International consensus on post-transplantation diabetes mellitus
- PMID: 38171510
- PMCID: PMC11024828
- DOI: 10.1093/ndt/gfad258
International consensus on post-transplantation diabetes mellitus
Abstract
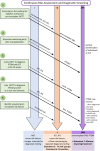
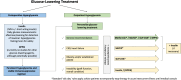
Post-transplantation diabetes mellitus (PTDM) remains a leading complication after solid organ transplantation. Previous international PTDM consensus meetings in 2003 and 2013 provided standardized frameworks to reduce heterogeneity in diagnosis, risk stratification and management. However, the last decade has seen significant advancements in our PTDM knowledge complemented by rapidly changing treatment algorithms for management of diabetes in the general population. In view of these developments, and to ensure reduced variation in clinical practice, a 3rd international PTDM Consensus Meeting was planned and held from 6-8 May 2022 in Vienna, Austria involving global delegates with PTDM expertise to update the previous reports. This update includes opinion statements concerning optimal diagnostic tools, recognition of prediabetes (impaired fasting glucose and/or impaired glucose tolerance), new mechanistic insights, immunosuppression modification, evidence-based strategies to prevent PTDM, treatment hierarchy for incorporating novel glucose-lowering agents and suggestions for the future direction of PTDM research to address unmet needs. Due to the paucity of good quality evidence, consensus meeting participants agreed that making GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) recommendations would be flawed. Although kidney-allograft centric, we suggest that these opinion statements can be appraised by the transplantation community for implementation across different solid organ transplant cohorts. Acknowledging the paucity of published literature, this report reflects consensus expert opinion. Attaining evidence is desirable to ensure establishment of optimized care for any solid organ transplant recipient at risk of, or who develops, PTDM as we strive to improve long-term outcomes.
Keywords: GLP-1 analogues; NODAT; SGLT2 inhibitors; metabolic syndrome; post-transplant diabetes mellitus.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
A.S. has received lecture fees from Chiesi and Napp Pharamceuticals, travel support from Sandoz, grant money from Chiesi and advisory board fees from Novartis. J.P. received lecture fees from Novartis, Chiesi and Sanofi. A.Kukla has received product support from Dexcom and is on the NovoNordisk Advisory Board. M.G. has received research support from Chiesi, lecture fees from Alexion, Astellas, AstraZeneca, Baxter, Bayer, Lilly and Novartis, advisory board fees from Alexion, Boehringer/Lilly and Chiesi, and travel support from Alexion, Astellas and Boehringer/Lilly. K.E. has received grant support from Chiesi, lecture fees from AstraZeneca, Alexion, Chiesi and Novartis, and advisory board fees from AstraZeneca, Alexion and Chiesi. M.Hecking served as a speaker and/or consultant for Astellas Pharma, AstraZeneca, Bayer, Eli Lilly, Fresenius Medical Care, Janssen-Cilag, Siemens Healthcare and Vifor, and received academic study support from Astellas Pharma, Boehringer Ingelheim, Eli Lilly, Nikkiso and Siemens Healthcare. M.Hornum received advisory board fee from AstraZeneca and Bayer, and travel support from AstraZeneca, and also served as speaker and moderator for AstraZeneca. N.M. has received travel support from NovoNordisk and Nordic Pharma and lecture fees from Sanofi. M.K. has received research support from AstraZeneca and Fit for Me, speaker and consulting fees from Lilly, Takeda, Ipsen and Sanofi, and travel support from Pfizer, Novo Nordisk, Merck, Ipsen, HRA Pharma and Boehringer-Ingelheim. A.P.J.V. has received speaker and advisory board fees from Novartis, Sandoz, Chiesi, Astellas, AstraZeneca and CSL Behring (all fees to employer). E.S. has received speaker fees from Amgen and Novartis, and travel support from Takeda and Astellas. M.C.H. has received speaker fees from AstraZeneca and Vifor. T.G.J. has received lecture fees from Boehring Ingelheim, AstraZeneca, NovoNordisk and Takeda, and advisory board fees from Bayer and Abbot Diagnostics. E.N. has received lecture fees from AstraZeneca. A.Kurnikowski, H.C., A.K.-W. and E.P. have no relevant disclosures to report
Figures
References
-
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care 2021;44:S15–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical