Modulation of tumor microenvironment by targeting histone acetylation in bladder cancer
- PMID: 38172127
- PMCID: PMC10764810
- DOI: 10.1038/s41420-023-01786-3
Modulation of tumor microenvironment by targeting histone acetylation in bladder cancer
Abstract
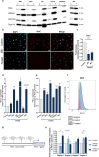
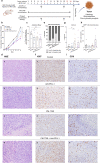
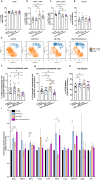
Alterations in the epigenetic machinery in both tumor and immune cells contribute to bladder cancer (BC) development, constituting a promising target as an alternative therapeutic option. Here, we have explored the effects of a novel histone deacetylase (HDAC) inhibitor CM-1758, alone or in combination with immune checkpoint inhibitors (ICI) in BC. We determined the antitumor effects of CM-1758 in various BC cell lines together with the induction of broad transcriptional changes, with focus on the epigenetic regulation of PD-L1. Using an immunocompetent syngeneic mouse model of metastatic BC, we studied the effects of CM-1758 alone or in combination with anti-PD-L1 not only on tumor cells, but also in the tumor microenvironment. In vitro, we found that CM-1758 has cytotoxic and cytostatic effects either by inducing apoptosis or cell cycle arrest in BC cells at low micromolar levels. PD-L1 is epigenetically regulated by histone acetylation marks and is induced after treatment with CM-1758. We also observed that treatment with CM-1758 led to an important delay in tumor growth and a higher CD8 + T cell tumor infiltration. Moreover, anti-PD-L1 alone or in combination with CM-1758 reprogramed macrophage differentiation towards a M1-like polarization state and increased of pro-inflammatory cytokines systemically, yielding potential further antitumor effects. Our results suggest the possibility of combining HDAC inhibitors with immunotherapies for the management of advanced metastatic BC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. Springer International Publishing, (2018).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

