N-glycan profiling of tissue samples to aid breast cancer subtyping
- PMID: 38172220
- PMCID: PMC10764792
- DOI: 10.1038/s41598-023-51021-3
N-glycan profiling of tissue samples to aid breast cancer subtyping
Abstract
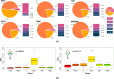
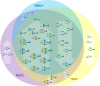
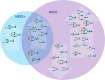
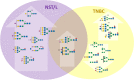
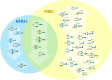
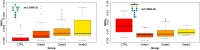
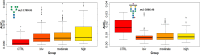
Breast cancer is a highly heterogeneous disease. Its intrinsic subtype classification for diagnosis and choice of therapy traditionally relies on the presence of characteristic receptors. Unfortunately, this classification is often not sufficient for precise prediction of disease prognosis and treatment efficacy. The N-glycan profiles of 145 tumors and 10 healthy breast tissues were determined using Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry. The tumor samples were classified into Mucinous, Lobular, No-Special-Type, Human Epidermal Growth Factor 2 + , and Triple-Negative Breast Cancer subtypes. Statistical analysis was conducted using the reproducibility-optimized test statistic software package in R, and the Wilcoxon rank sum test with continuity correction. In total, 92 N-glycans were detected and quantified, with 59 consistently observed in over half of the samples. Significant variations in N-glycan signals were found among subtypes. Mucinous tumor samples exhibited the most distinct changes, with 28 significantly altered N-glycan signals. Increased levels of tri- and tetra-antennary N-glycans were notably present in this subtype. Triple-Negative Breast Cancer showed more N-glycans with additional mannose units, a factor associated with cancer progression. Individual N-glycans differentiated Human Epidermal Growth Factor 2 + , No-Special-Type, and Lobular cancers, whereas lower fucosylation and branching levels were found in N-glycans significantly increased in Luminal subtypes (Lobular and No-Special-Type tumors). Clinically normal breast tissues featured a higher abundance of signals corresponding to N-glycans with bisecting moiety. This research confirms that histologically distinct breast cancer subtypes have a quantitatively unique set of N-glycans linked to clinical parameters like tumor size, proliferative rate, lymphovascular invasion, and metastases to lymph nodes. The presented results provide novel information that N-glycan profiling could accurately classify human breast cancer samples, offer stratification of patients, and ongoing disease monitoring.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Stewart SF, Slodkowska-Barabasz J, McGeagh L, Moon Z, Brett J, Wells M, et al. Development of the HT&Me intervention to support women with breast cancer to adhere to adjuvant endocrine therapy and improve quality of life. Breast. 2023;70:32–40. doi: 10.1016/j.breast.2023.05.007. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- MH CZ - DRO (MMCI, 00209805)/the Ministry of Health Development of Research Organization
- CZ.02.1.01/0.0/0.0/16_019/0000868/the European Regional Development Fund - Project ENOCH
- LM2018125/BBMRI-CZ
- LM2023042/CIISB, Instruct-CZ Centre of Instruct-ERIC EU consortium, funded by MEYS CR infrastructure project
- No. CZ.02.1.01/0.0/0.0/18_046/0015974/European Regional Development Fund-Project "UP CIISB"
LinkOut - more resources
Full Text Sources

