Characterization, antibacterial, and cytotoxic activities of silver nanoparticles using the whole biofilm layer as a macromolecule in biosynthesis
- PMID: 38172225
- PMCID: PMC10764356
- DOI: 10.1038/s41598-023-50548-9
Characterization, antibacterial, and cytotoxic activities of silver nanoparticles using the whole biofilm layer as a macromolecule in biosynthesis
Abstract
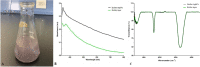
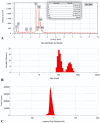
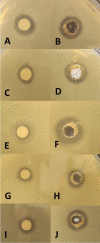
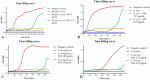
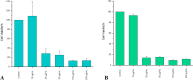
Recently, multi-drug resistant (MDR) bacteria are responsible for a large number of infectious diseases that can be life-threatening. Globally, new approaches are targeted to solve this essential issue. This study aims to discover novel antibiotic alternatives by using the whole components of the biofilm layer as a macromolecule to synthesize silver nanoparticles (AgNPs) as a promising agent against MDR. In particular, the biosynthesized biofilm-AgNPs were characterized using UV-Vis spectroscopy, electron microscopes, Energy Dispersive X-ray (EDX), zeta sizer and potential while their effect on bacterial strains and normal cell lines was identified. Accordingly, biofilm-AgNPs have a lavender-colored solution, spherical shape, with a size range of 20-60 nm. Notably, they have inhibitory effects when used on various bacterial strains with concentrations ranging between 12.5 and 25 µg/mL. In addition, they have an effective synergistic effect when combined with phage ZCSE9 to inhibit and kill Salmonella enterica with a concentration of 3.1 µg/mL. In conclusion, this work presents a novel biosynthesis preparation of AgNPs using biofilm for antibacterial purposes to reduce the possible toxicity by reducing the MICs using phage ZCSE9.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



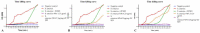




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

