Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL)
- PMID: 38172296
- PMCID: PMC10810941
- DOI: 10.1007/s00134-023-07271-w
Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL)
Erratum in
-
Correction: Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL).Intensive Care Med. 2024 Apr;50(4):614-615. doi: 10.1007/s00134-024-07357-z. Intensive Care Med. 2024. PMID: 38436728 Free PMC article. No abstract available.
Abstract
Purpose: Ilofotase alfa is a human recombinant alkaline phosphatase with reno-protective effects that showed improved survival and reduced Major Adverse Kidney Events by 90 days (MAKE90) in sepsis-associated acute kidney injury (SA-AKI) patients. REVIVAL, was a phase-3 trial conducted to confirm its efficacy and safety.
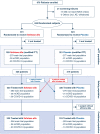
Methods: In this international double-blinded randomized-controlled trial, SA-AKI patients were enrolled < 72 h on vasopressor and < 24 h of AKI. The primary endpoint was 28-day all-cause mortality. The main secondary endpoint was MAKE90, other secondary endpoints were (i) days alive and free of organ support through day 28, (ii) days alive and out of the intensive care unit (ICU) through day 28, and (iii) time to death through day 90. Prior to unblinding, the statistical analysis plan was amended, including an updated MAKE90 definition.
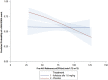
Results: Six hundred fifty patients were treated and analyzed for safety; and 649 for efficacy data (ilofotase alfa n = 330; placebo n = 319). The observed mortality rates in the ilofotase alfa and placebo groups were 27.9% and 27.9% at 28 days, and 33.9% and 34.8% at 90 days. The trial was stopped for futility on the primary endpoint. The observed proportion of patients with MAKE90A and MAKE90B were 56.7% and 37.4% in the ilofotase alfa group vs. 64.6% and 42.8% in the placebo group. Median [interquartile range (IQR)] days alive and free of organ support were 17 [0-24] and 14 [0-24], number of days alive and discharged from the ICU through day 28 were 15 [0-22] and 10 [0-22] in the ilofotase alfa and placebo groups, respectively. Adverse events were reported in 67.9% and 75% patients in the ilofotase and placebo group.
Conclusion: Among critically ill patients with SA-AKI, ilofotase alfa did not improve day 28 survival. There may, however, be reduced MAKE90 events. No safety concerns were identified.
Trial registration: ClinicalTrials.gov NCT04411472.
Keywords: Acute kidney injury; Chronic kidney disease; MAKE90; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
PP has received travel reimbursements and consulting fees from AM-Pharma in relation to his role as PI for REVIVAL, and consulting fees from Adrenomed, EBI Paion, Sphingotec, and 4Teen4 outside the submitted work. DCA has received consulting fees from AM-Pharma. KB, AM-Pharma BV, The Netherlands. RB has received consulting fees and research support from AM-Pharma, Baxter, Paion, Viatris, Jafron Biomedical, and CSL Behring. EvdB, AM-Pharma BV, The Netherlands. JB, AM-Pharma BV, The Netherlands. MHB has received consulting fees from AM-Pharma in relation to his role for REVIVAL and has conducted contract research for Inotrem outside of the submitted work. KD has received consulting fees from AM-Pharma. CJD reports no conflicts of interest. RF has received consulting fees from AM-Pharma. BF has received consulting fees from AM-Pharma as a member of the REVIVAL steering committee, and consulting fees from Inotrem, Aridis, and Enlivex outside the submitted work. HG reports funding from various companies in the form of research grants to (and administered by) Aarhus University or Aarhus University Hospital. HG has received support for attending meetings by Baxter A/S. UGP reports no conflicts of interest. EH has received a travel grant from AM-Pharma. SI reports no conflicts of interest. Michael Joannidis has received honoraria or research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius, Takeda, Sanofi and Novartis. JAK discloses fees paid by AM-Pharma in relation to his role as national PI for REVIVAL and is currently a full-time employee of Spectral Medical. KL has been a member of the REVIVAL Steering Committee for AM Pharma. She has been a consultant/member of the DSMB for Seastar, Novartis, BOA Medical, Baxter, and Biomerieux, and she holds stock in Amgen. MM has received lecture fees from Baxter and Fresenius Medical Care. RM reports honoraria for consulting from Baxter, Biomerieux, Mallinckrodt, GE Healthcare, Sanofi, Abiomed, NovaBiomed, Renasym, and advisory board reimbursements from AM Pharma, Renibus, Alexion, Novartis, and Guard. SM reports no conflicts of interest. PTM has received consulting fees from AM-Pharma (for Clinical Trial Steering Committee activities), Novartis, Renibus Therapeutics, and Alexion. AN reports an unrestricted grant from Baxter to support the renal substudy of the TAME trial. MO has received speaker honoraria from Fresenius Medical, Baxter and Biomerieux; her institution received research funding from Baxter, Fresenius Medical, Biomerieux, and LaJolla Pharma. CS reports no conflicts of interest. PV reports no conflicts of interest. MW, AM-Pharma BV, The Netherlands. PJY has received consulting fees from AM Pharma and from Baxter Healthcare Pty. AZ has received consulting fees from Astute-Biomerieux, Baxter, Bayer, Novartis, Guard Therapeutics, AM Pharma, Paion, Renibus, Fresenius, research funding from Astute-Biomerieux, Fresenius, Baxter, and speakers fees from Astute-Biomerieux, Fresenius, Baxter.
Figures
References
-
- Pickkers P, Angus DC, Arend J, Bellomo R, van den Berg E, Bernholz J, Bestle M, Broglio K, Carlsen J, Doig CJ, Ferrer R, Joannidis M, Francois B, Doi K, Kellum JA, Laterre PF, Liu K, Mehta RL, Murray PT, Ostermann M, Pettilä V, Richards S, Young P, Zarbock A, Kjølbye AL. Study protocol of a randomised, double-blind, placebo-controlled, two-arm parallel-group, multi-centre phase 3 pivotal trial to investigate the efficacy and safety of recombinant human alkaline phosphatase for treatment of patients with sepsis-associated acute kidney injury. BMJ Open. 2023;13:e065613. doi: 10.1136/bmjopen-2022-065613. - DOI - PMC - PubMed
-
- Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. - DOI - PMC - PubMed
-
- Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, Kashani K, Koyner JL, Pannu N, Meersch M, Reis T, Rimmelé T, Bagshaw SM, Bellomo R, Cantaluppi V, Deep A, De Rosa S, Perez-Fernandez X, Husain-Syed F, Kane-Gill SL, Kelly Y, Mehta RL, Murray PT, Ostermann M, Prowle J, Ricci Z, See EJ, Schneider A, Soranno DE, Tolwani A, Villa G, Ronco C, Forni LG. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 2023;19:401–417. doi: 10.1038/s41581-023-00683-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

