A novel antibiotic class targeting the lipopolysaccharide transporter
- PMID: 38172634
- PMCID: PMC10794144
- DOI: 10.1038/s41586-023-06873-0
A novel antibiotic class targeting the lipopolysaccharide transporter
Erratum in
-
Author Correction: A novel antibiotic class targeting the lipopolysaccharide transporter.Nature. 2024 Jul;631(8022):E17. doi: 10.1038/s41586-024-07641-4. Nature. 2024. PMID: 38992182 Free PMC article. No abstract available.
Abstract
Carbapenem-resistant Acinetobacter baumannii (CRAB) has emerged as a major global pathogen with limited treatment options1. No new antibiotic chemical class with activity against A. baumannii has reached patients in over 50 years1. Here we report the identification and optimization of tethered macrocyclic peptide (MCP) antibiotics with potent antibacterial activity against CRAB. The mechanism of action of this molecule class involves blocking the transport of bacterial lipopolysaccharide from the inner membrane to its destination on the outer membrane, through inhibition of the LptB2FGC complex. A clinical candidate derived from the MCP class, zosurabalpin (RG6006), effectively treats highly drug-resistant contemporary isolates of CRAB both in vitro and in mouse models of infection, overcoming existing antibiotic resistance mechanisms. This chemical class represents a promising treatment paradigm for patients with invasive infections due to CRAB, for whom current treatment options are inadequate, and additionally identifies LptB2FGC as a tractable target for antimicrobial drug development.
© 2024. The Author(s).
Conflict of interest statement
C.Z., P. Mattei, K.B., L.W., J.-M.A., C. Bucher, C.T., A.A., K.E.A., C. Bieniossek, C. Bissantz, F.B., C.C., T.C., F.D., P.D.G., P.d.C., D.D., P.D., F.G.-A., A.H., M.L., S.L., P. Misson, S.R., A.S., S.S., P.S., T.S., A.T., S.Z., J.A.T.Y., M.A.L. and K.A.B. are current or former employees of F. Hoffmann-La Roche. C.Z., K.B., A.A., A.S. and T.S. are listed as inventors on the approved United States Patent US10,030,047, which covers the molecules RO7036668, RO7075573 and RO7202110. P. Mattei, K.B., P.D.G., P.S. and T.S. are listed as inventors on the pending patent application US2019/0321440, which covers the molecule zosurabalpin.
Figures
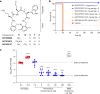

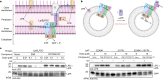




Comment in
-
A new class of antibiotics is cause for cautious celebration - but the economics must be fixed.Nature. 2024 Jan;625(7993):7. doi: 10.1038/d41586-023-04086-z. Nature. 2024. PMID: 38172365 No abstract available.
-
Macrocyclic peptides thwart Gram-negative bacteria.Nat Rev Drug Discov. 2024 Mar;23(3):171. doi: 10.1038/d41573-024-00021-7. Nat Rev Drug Discov. 2024. PMID: 38316949 No abstract available.
References
-
- Centers for Disease Control and Prevention. 2019 AR Threats Report. CDChttps://www.cdc.gov/DrugResistance/Biggest-Threats.html (2019).
-
- Cassini A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

