Spotting disease disrupts the microbiome of infected purple sea urchins, Strongylocentrotus purpuratus
- PMID: 38172649
- PMCID: PMC10765733
- DOI: 10.1186/s12866-023-03161-9
Spotting disease disrupts the microbiome of infected purple sea urchins, Strongylocentrotus purpuratus
Abstract
Background: Spotting disease infects a variety of sea urchin species across many different marine locations. The disease is characterized by discrete lesions on the body surface composed of discolored necrotic tissue that cause the loss of all surface appendages within the lesioned area. A similar, but separate disease of sea urchins called bald sea urchin disease (BSUD) has overlapping symptoms with spotting disease, resulting in confusions in distinguishing the two diseases. Previous studies have focus on identifying the underlying causative agent of spotting disease, which has resulted in the identification of a wide array of pathogenic bacteria that vary based on location and sea urchin species. Our aim was to investigate the spotting disease infection by characterizing the microbiomes of the animal surface and various tissues.
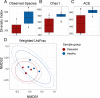
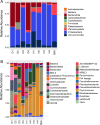
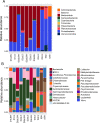
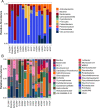
Results: We collected samples of the global body surface, the lesion surface, lesioned and non-lesioned body wall, and coelomic fluid, in addition to samples from healthy sea urchins. 16S rRNA gene was amplified and sequenced from the genomic DNA. Results show that the lesions are composed mainly of Cyclobacteriaceae, Cryomorphaceae, and a few other taxa, and that the microbial composition of lesions is the same for all infected sea urchins. Spotting disease also alters the microbial composition of the non-lesioned body wall and coelomic fluid of infected sea urchins. In our closed aquarium systems, sea urchins contracted spotting disease and BSUD separately and therefore direct comparisons could be made between the microbiomes from diseased and healthy sea urchins.
Conclusion: Results show that spotting disease and BSUD are separate diseases with distinct symptoms and distinct microbial compositions.
Keywords: 16S rRNA; Disease; Infection; Lesion; Microbiome; Pathogenic.
© 2023. The Author(s).
Conflict of interest statement
Competing interests. The authors declare no competing interests.
Figures




References
-
- Shimizu M, Takaya Y, Ohsaki S, Kawamata K. Gross and histopathological signs of the spotting disease in the sea urchin Strongylocentrotus intermedius. Fish Sci. 1995;61:608–13. 10.1016/S0167-9309(07)80073-1
-
- Tajima K, Hirano T, Shimizu M, Ezura Y. Isolation and pathogenicity of the causative bacterium of spotting disease of sea urchin Strongylocentrotus intermedius. Fish Sci. 1997;63(2):249–52. 10.2331/fishsci.63.249
-
- Zhang W, Lv Z, Li C, Sun Y, Jiang H, Zhao M, Zhao X, Shao Y, Chang Y. Transcriptome profiling reveals key roles of phagosome and NOD-like receptor pathway in spotting diseased Strongylocentrotus intermedius. Fish Shellfish Immunol. 2019;84:521–31. 10.1016/j.fsi.2018.10.042 - PubMed
-
- Li R, Dang H, Huang Y, Quan Z, Jiang H, Zhang W, Ding J. Vibrio coralliilyticus as an agent of red spotting disease in the sea urchin Strongylocentrotus intermedius. Aquac Rep. 2020;16:100244. 10.1016/j.aqrep.2019.100244
-
- Wang L, He B, Chang Y, Ding J. Characterization of the bacterial community associated with red spotting disease of the echinoid Strongylocentrotus intermedius. Aquaculture. 2020;529:735606. 10.1016/j.aquaculture.2020.735606
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

