Adverse events in single-arm clinical trials with non-fatal time-to-event efficacy endpoint: from clinical questions to methods for statistical analysis
- PMID: 38172810
- PMCID: PMC10765745
- DOI: 10.1186/s12874-023-02123-z
Adverse events in single-arm clinical trials with non-fatal time-to-event efficacy endpoint: from clinical questions to methods for statistical analysis
Abstract
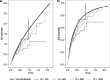
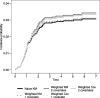
Background: In any single-arm trial on novel treatments, assessment of toxicity plays an important role as occurrence of adverse events (AEs) is relevant for application in clinical practice. In the presence of a non-fatal time-to-event(s) efficacy endpoint, the analysis should be broadened to consider AEs occurrence in time. The AEs analysis could be tackled with two approaches, depending on the clinical question of interest. Approach 1 focuses on the occurrence of AE as first event. Treatment ability to protect from the efficacy endpoint event(s) has an impact on the chance of observing AEs due to competing risks action. Approach 2 considers how treatment affects the occurrence of AEs in the potential framework where the efficacy endpoint event(s) could not occur.
Methods: In the first part of the work we review the strategy of analysis for these two approaches. We identify theoretical quantities and estimators consistent with the following features: (a) estimators should address for the presence of right censoring; (b) theoretical quantities and estimators should be functions of time. In the second part of the work we propose the use of alternative methods (regression models, stratified Kaplan-Meier curves, inverse probability of censoring weighting) to relax the assumption of independence between the potential times to AE and to event(s) in the efficacy endpoint for addressing Approach 2.
Results: We show through simulations that the proposed methods overcome the bias due to the dependence between the two potential times and related to the use of standard estimators.
Conclusions: We demonstrated through simulations that one can handle patients selection in the risk sets due to the competing event, and thus obtain conditional independence between the two potential times, adjusting for all the observed covariates that induce dependence.
Keywords: Adverse events; Competing risks; Dependent censoring; IPCW; Survival analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
The net benefit for time-to-event outcome in oncology clinical trials with treatment switching.Clin Trials. 2023 Dec;20(6):670-680. doi: 10.1177/17407745231186081. Epub 2023 Jul 16. Clin Trials. 2023. PMID: 37455538
-
Survival analysis for AdVerse events with VarYing follow-up times (SAVVY)-estimation of adverse event risks.Trials. 2021 Jun 29;22(1):420. doi: 10.1186/s13063-021-05354-x. Trials. 2021. PMID: 34187527 Free PMC article.
-
Statistical issues in the analysis of adverse events in time-to-event data.Pharm Stat. 2016 Jul;15(4):297-305. doi: 10.1002/pst.1739. Epub 2016 Mar 1. Pharm Stat. 2016. PMID: 26929180
-
Considerations for analysis of time-to-event outcomes subject to competing risks in veterinary clinical studies.J Vet Cardiol. 2018 Jun;20(3):143-153. doi: 10.1016/j.jvc.2018.03.001. Epub 2018 Apr 18. J Vet Cardiol. 2018. PMID: 29680637 Review.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
Cited by
-
Xietu Hemu Prescription Improves Metabolic Dysfunction-Associated Steatotic Liver Disease: A Real-World Cohort Study.J Multidiscip Healthc. 2025 Jul 29;18:4377-4389. doi: 10.2147/JMDH.S522519. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40756613 Free PMC article.
References
-
- Parasole R, Valsecchi MG, Silvestri D, Locatelli F, Barisone E, Petruzziello F, et al. Correspondence: Osteonecrosis in childhood acute lymphoblastic leukemia: a retrospective cohort study of the Italian Association of Pediatric Haemato-Oncology (AIEOP) Blood Cancer J. 2018;8(12):115. doi: 10.1038/s41408-018-0150-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

