TET2-mediated ECM1 hypomethylation promotes the neovascularization in active proliferative diabetic retinopathy
- PMID: 38172938
- PMCID: PMC10765922
- DOI: 10.1186/s13148-023-01619-1
TET2-mediated ECM1 hypomethylation promotes the neovascularization in active proliferative diabetic retinopathy
Abstract
Background: Studies have shown that tet methylcytosine dioxygenase 2 (TET2) is highly expressed in diabetic retinopathy (DR), which reduces the DNA methylation of downstream gene promoters and activates the transcription. Abnormally expressed TET2 and downstream genes in a high-glucose environment are associated with retinal capillary leakage and neovascularization. Here, we investigated the downstream genes of TET2 and its potential association with neovascularization in proliferative diabetic retinopathy (PDR).
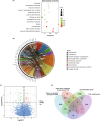
Methods: GSE60436, GSE57362, and GSE158333 datasets were analyzed to identify TET2-related hypomethylated and upregulated genes in PDR. Gene expression and promoter methylation of these genes under high glucose treatment were verified. Moreover, TET2 knockdown was used to assess its impact on tube formation and migration in human retinal microvascular endothelial cells (HRMECs), as well as its influence on downstream genes.
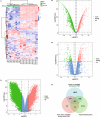
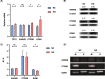
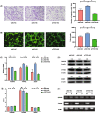
Results: Our analysis identified three key genes (PARVB, PTPRE, ECM1) that were closely associated with TET2 regulation. High glucose-treated HRMECs exhibited increased expression of TET2 and ECM1 while decreasing the promoter methylation level of ECM1. Subsequently, TET2 knockdown led to decreased migration ability and tube formation function of HRMECs. We further found a decreased expression of PARVB, PTPRE, and ECM1, accompanied by an increase in the promoter methylation of ECM1.
Conclusions: Our findings indicate the involvement of dysregulated TET2 expression in neovascularization by regulating the promoter methylation and transcription of downstream genes (notably ECM1), eventually leading to PDR. The TET2-induced hypomethylation of downstream gene promoters represents a potential therapeutic target and offers a novel perspective on the mechanism underlying neovascularization in PDR.
Keywords: DNA methylation; ECM1; Epigenetics; Neovascularization; Proliferative diabetic retinopathy (PDR); TET2.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The role of TET2-mediated ROBO4 hypomethylation in the development of diabetic retinopathy.J Transl Med. 2023 Jul 10;21(1):455. doi: 10.1186/s12967-023-04310-4. J Transl Med. 2023. PMID: 37430272 Free PMC article.
-
The palliative effects of folic acid on retinal microvessels in diabetic retinopathy via regulating the metabolism of DNA methylation and hydroxymethylation.Bioengineered. 2021 Dec;12(2):10766-10774. doi: 10.1080/21655979.2021.2003924. Bioengineered. 2021. PMID: 34874218 Free PMC article.
-
Identification of the aberrantly methylated differentially expressed genes in proliferative diabetic retinopathy.Exp Eye Res. 2020 Oct;199:108141. doi: 10.1016/j.exer.2020.108141. Epub 2020 Jul 25. Exp Eye Res. 2020. PMID: 32721427
-
TET proteins and 5-methylcytosine oxidation in hematological cancers.Immunol Rev. 2015 Jan;263(1):6-21. doi: 10.1111/imr.12239. Immunol Rev. 2015. PMID: 25510268 Free PMC article. Review.
-
Candidate genes for proliferative diabetic retinopathy.Biomed Res Int. 2013;2013:540416. doi: 10.1155/2013/540416. Epub 2013 Aug 27. Biomed Res Int. 2013. PMID: 24066292 Free PMC article. Review.
Cited by
-
Distinct types of protein modifications in diabetic endothelial dysfunction.Cardiovasc Diabetol. 2025 Jul 14;24(1):287. doi: 10.1186/s12933-025-02836-z. Cardiovasc Diabetol. 2025. PMID: 40660266 Free PMC article. Review.
-
Regulation role of miR-204 on SIRT1/VEGF in metabolic memory induced by high glucose in human retinal pigment epithelial cells.Int J Ophthalmol. 2024 Jul 18;17(7):1232-1237. doi: 10.18240/ijo.2024.07.06. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39026923 Free PMC article.
-
Traversing the epigenetic landscape: DNA methylation from retina to brain in development and disease.Front Cell Neurosci. 2024 Nov 29;18:1499719. doi: 10.3389/fncel.2024.1499719. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39678047 Free PMC article. Review.
-
Atherosclerosis in diabetes mellitus: novel mechanisms and mechanism-based therapeutic approaches.Nat Rev Cardiol. 2025 Jul;22(7):482-496. doi: 10.1038/s41569-024-01115-w. Epub 2025 Jan 13. Nat Rev Cardiol. 2025. PMID: 39805949 Review.
-
DNA hypermethylation of PLTP mediated by DNMT3B aggravates vascular dysfunction in diabetic retinopathy via the AKT/GSK3β signaling pathway.Clin Epigenetics. 2025 May 17;17(1):82. doi: 10.1186/s13148-025-01874-4. Clin Epigenetics. 2025. PMID: 40380281 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

