Histone acetylation: a key determinant of acquired cisplatin resistance in cancer
- PMID: 38172984
- PMCID: PMC10765630
- DOI: 10.1186/s13148-023-01615-5
Histone acetylation: a key determinant of acquired cisplatin resistance in cancer
Abstract
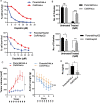
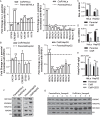
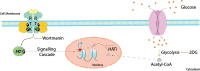
Cisplatin is an alkylating class of chemotherapeutic drugs used to treat cancer patients. However, cisplatin fails in long-term treatment, and drug resistance is the primary reason for tumor recurrence. Hence, understanding the mechanism of acquirement of chemoresistance is essential for developing novel combination therapeutic approaches. In this study, in vitro cisplatin-resistant cancer cell line models were developed. Gene ontology and GSEA of differentially expressed genes between parental and resistant cells suggest that PI3K-AKT signaling, central carbon metabolism, and epigenetic-associated phenomenon alter in cisplatin-resistant cells. Further, the data showed that increased glucose transport, alteration in the activity of histone-modifying enzymes, and acetyl-CoA levels in resistant cells paralleled an increase in global histone acetylation. Enrichment of histone acetylation on effectors of PI3K-AKT and glycolysis pathway provides evidence of epigenetic regulation of the key molecules in drug resistance. Moreover, cisplatin treatment to resistant cells showed no significant changes in histone acetylation marks since drug treatment alters cell epigenome. In continuation, targeting PI3K-AKT signaling and glycolysis leads to alteration in histone acetylation levels and re-sensitization of resistant cells to chemo-drug. The data provide evidence of histone acetylation's importance in regulating pathways and cisplatin-resistant cells' cell survival. Our study paves the way for new approaches for developing personalized therapies in affecting metabolic pathways and epigenetic changes to achieve better outcomes for targeting drug-resistant cells.
Keywords: 2DG; Cisplatin; Hyperacetylation; PI3K; Resistance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

