Phosphatidylserine enriched with polyunsaturated n-3 fatty acid supplementation for attention-deficit hyperactivity disorder in children and adolescents with epilepsy: A randomized placebo-controlled trial
- PMID: 38173190
- PMCID: PMC10984292
- DOI: 10.1002/epi4.12892
Phosphatidylserine enriched with polyunsaturated n-3 fatty acid supplementation for attention-deficit hyperactivity disorder in children and adolescents with epilepsy: A randomized placebo-controlled trial
Abstract
Background: Attention-deficit hyperactivity disorder (ADHD) is a frequent comorbidity in children with epilepsy, which management mostly relies on the usual treatments of ADHD, especially methylphenidate. Supplementation with polyunsaturated n-3 Fatty Acid (PUFA) has been proposed as an alternative therapeutic approach in ADHD without epilepsy but has never been evaluated in epilepsy-associated ADHD.
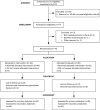
Methods: A multicenter double blind randomized placebo-controlled trial evaluating supplementation with PUFA, in eicosapentaenoic- and docosahexaenoic-acid form, conjugated to a phospholipid vector (PS-Omega3) in children aged >6 and <16-years old, and suffering from any type of epilepsy and ADHD (inattentive or combined type) according to DSM-V. After a 4-week baseline period, patients were allocated (1:1) either to placebo group or to PS-Omega 3 group and entered a 12 week-double-blind treatment period which was followed by a 12 week-open-label treatment period. The primary outcome was the reduction of the ADHD-rating scale IV attention-deficit subscore after 12 weeks of treatment.
Results: The study was stopped early because of lack of eligible participants and the expected sample size was not reached. Seventy-four patients were randomized, 44 in PS-Omega3, and 30 in the placebo group. The reduction after 12 weeks of treatment in the inattention subscore of the ADHD-IV scale was -1.57 in the PS-Omega3 group, and -2.90 in the placebo group (p = 0.33, α = 5%). Results were similar after 24 weeks of treatment and for all other ADHD-related secondary outcomes, with no difference between placebo and PS-Omega3.
Conclusion: Our study remaining underpowered, no formal conclusion about the effect of Ps-Omega3 could be drawn. However, our data strongly suggested that the PS-Omega 3 formulation used in the current study did not improve ADHD symptoms in children with epilepsy.
Plain language summary: Supplementation with polyunsaturated n-3 Fatty Acid (PUFA) has been proposed in ADHD but has never been evaluated in patients with both epilepsy and ADHD. To address this issue, we conducted a multicenter double blind randomized placebo-controlled trial evaluating supplementation with PUFA in children with epilepsy and ADHD. The study was stopped early because of lack of eligible participants, hampering formal conclusion. However, the evolution of the ADHD symptoms at 12 and 24 weeks did not differ between placebo and PUFA supplementation, strongly suggesting that PUFA did not improve ADHD symptoms in children with epilepsy.
Keywords: ADHD; epilepsy; omega 3; polyunsaturated n‐3 fatty acid.
© 2024 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
SR received speaker and/or consulting fees from UCB Pharma, Angelini Pharma, EISAI, Livanova, JAZZ Pharma, Medtronic, Zogenix. AA, in agreement with his Institution, has served as principal investigator or member of DMCs in clinical trials for Eisai, UCB, GW Pharma; received consulting fees from Jazz, Zogenix, Eisai, Takeda, Biocodex, Encoded Therapeutics; unrestricted research grants from, UCB, Caixa Foundation and Jazz and academic research grants from EJP‐RD and the EU. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Similar articles
-
The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: a double-blind placebo-controlled trial, followed by an open-label extension.Eur Psychiatry. 2012 Jul;27(5):335-42. doi: 10.1016/j.eurpsy.2011.05.004. Epub 2011 Jul 31. Eur Psychiatry. 2012. PMID: 21807480 Clinical Trial.
-
Safety of phosphatidylserine containing omega3 fatty acids in ADHD children: a double-blind placebo-controlled trial followed by an open-label extension.Eur Psychiatry. 2013 Aug;28(6):386-91. doi: 10.1016/j.eurpsy.2012.11.001. Epub 2013 Jan 9. Eur Psychiatry. 2013. PMID: 23312676 Clinical Trial.
-
A randomized controlled trial investigating the effects of PCSO-524, a patented oil extract of the New Zealand green lipped mussel (Perna canaliculus), on the behaviour, mood, cognition and neurophysiology of children and adolescents (aged 6-14 years) experiencing clinical and sub-clinical levels of hyperactivity and inattention: study protocol ACTRN12610000978066.Nutr J. 2013 Jul 16;12:100. doi: 10.1186/1475-2891-12-100. Nutr J. 2013. PMID: 23866813 Free PMC article. Clinical Trial.
-
Supplementation with polyunsaturated fatty acids (PUFAs) in the management of attention deficit hyperactivity disorder (ADHD).Nutr Health. 2018 Dec;24(4):279-284. doi: 10.1177/0260106018772170. Epub 2018 Jun 19. Nutr Health. 2018. PMID: 29921155 Free PMC article. Review.
-
Which polyunsaturated fatty acids are active in children with attention-deficit hyperactivity disorder receiving PUFA supplementation? A fatty acid validated meta-regression analysis of randomized controlled trials.Prostaglandins Leukot Essent Fatty Acids. 2014 May;90(5):179-89. doi: 10.1016/j.plefa.2014.01.004. Epub 2014 Feb 3. Prostaglandins Leukot Essent Fatty Acids. 2014. PMID: 24560325 Review.
Cited by
-
Treatment Approaches for Psychiatric and Cognitive Comorbidities in Pediatric Epilepsy: A Systematic Review of Clinical Trials.Cureus. 2025 Jun 3;17(6):e85300. doi: 10.7759/cureus.85300. eCollection 2025 Jun. Cureus. 2025. PMID: 40612818 Free PMC article. Review.
References
-
- Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. - PubMed
-
- Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. - PubMed
-
- Sherman EM, Slick DJ, Connolly MB, Eyrl KL. ADHD, neurological correlates and health‐related quality of life in severe pediatric epilepsy. Epilepsia. 2007;48:1083–1091. - PubMed
-
- Auvin S, Wirrell E, Donald KA, Berl M, Hartmann H, Valente KD, et al. Systematic review of the screening, diagnosis, and management of ADHD in children with epilepsy. Consensus paper of the task force on comorbidities of the ILAE pediatric commission. Epilepsia. 2018;59:1867–1880. - PubMed
-
- Rheims S, Auvin S. Attention deficit/hyperactivity disorder and epilepsy. Curr Opin Neurol. 2021;34:219–225. - PubMed