DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
- PMID: 38173372
- PMCID: PMC10850270
- DOI: 10.4093/dmj.2022.0367
DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
Abstract
Backgruound: Nonalcoholic steatohepatitis (NASH) is a liver disease caused by obesity that leads to hepatic lipoapoptosis, resulting in fibrosis and cirrhosis. However, the mechanism underlying NASH is largely unknown, and there is currently no effective therapeutic agent against it. DWN12088, an agent used for treating idiopathic pulmonary fibrosis, is a selective prolyl-tRNA synthetase (PRS) inhibitor that suppresses the synthesis of collagen. However, the mechanism underlying the hepatoprotective effect of DWN12088 is not clear. Therefore, we investigated the role of DWN12088 in NASH progression.
Methods: Mice were fed a chow diet or methionine-choline deficient (MCD)-diet, which was administered with DWN12088 or saline by oral gavage for 6 weeks. The effects of DWN12088 on NASH were evaluated by pathophysiological examinations, such as real-time quantitative reverse transcription polymerase chain reaction, immunoblotting, biochemical analysis, and immunohistochemistry. Molecular and cellular mechanisms of hepatic injury were assessed by in vitro cell culture.
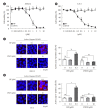
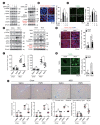
Results: DWN12088 attenuated palmitic acid (PA)-induced lipid accumulation and lipoapoptosis by downregulating the Rho-kinase (ROCK)/AMP-activated protein kinase (AMPK)/sterol regulatory element-binding protein-1c (SREBP-1c) and protein kinase R-like endoplasmic reticulum kinase (PERK)/α subunit of eukaryotic initiation factor 2 (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP-homologous protein (CHOP) signaling cascades. PA increased but DWN12088 inhibited the phosphorylation of nuclear factor-κB (NF-κB) p65 (Ser536, Ser276) and the expression of proinflammatory genes. Moreover, the DWN12088 inhibited transforming growth factor β (TGFβ)-induced pro-fibrotic gene expression by suppressing TGFβ receptor 1 (TGFβR1)/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) axis signaling. In the case of MCD-diet-induced NASH, DWN12088 reduced hepatic steatosis, inflammation, and lipoapoptosis and prevented the progression of fibrosis.
Conclusion: Our findings provide new insights about DWN12088, namely that it plays an important role in the overall improvement of NASH. Hence, DWN12088 shows great potential to be developed as a new integrated therapeutic agent for NASH.
Keywords: Idiopathic pulmonary fibrosis; Inflammation; Non-alcoholic fatty liver disease; Palmitic acid; Prolyl tRNA synthetase; Transforming growth factor beta.
Conflict of interest statement
Drug Discovery Center, Daewoong Pharmaceutical provided us with DWN12088 for this study. No potential conflict of interest relevant to this article was reported.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

