Genetic landscape of Parkinson's disease and related diseases in Luxembourg
- PMID: 38173558
- PMCID: PMC10761438
- DOI: 10.3389/fnagi.2023.1282174
Genetic landscape of Parkinson's disease and related diseases in Luxembourg
Abstract
Objectives: To explore the genetic architecture of PD in the Luxembourg Parkinson's Study including cohorts of healthy people and patients with Parkinson's disease (PD) and atypical parkinsonism (AP).
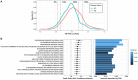
Methods: 809 healthy controls, 680 PD and 103 AP were genotyped using the Neurochip array. We screened and validated rare single nucleotide variants (SNVs) and copy number variants (CNVs) within seven PD-causing genes (LRRK2, SNCA, VPS35, PRKN, PARK7, PINK1 and ATP13A2). Polygenic risk scores (PRSs) were generated using the latest genome-wide association study for PD. We then estimated the role of common variants in PD risk by applying gene-set-specific PRSs.
Results: We identified 60 rare SNVs in seven PD-causing genes, nine of which were pathogenic in LRRK2, PINK1 and PRKN. Eleven rare CNVs were detected in PRKN including seven duplications and four deletions. The majority of PRKN SNVs and CNVs carriers were heterozygous and not differentially distributed between cases and controls. The PRSs were significantly associated with PD and identified specific molecular pathways related to protein metabolism and signal transduction as drivers of PD risk.
Conclusion: We performed a comprehensive genetic characterization of the deep-phenotyped individuals of the Luxembourgish Parkinson's Study. Heterozygous SNVs and CNVs in PRKN were not associated with higher PD risk. In particular, we reported novel digenic variants in PD related genes and rare LRRK2 SNVs in AP patients. Our findings will help future studies to unravel the genetic complexity of PD.
Keywords: Luxembourg; Parkinson’s disease; copy number variants; genetics; polygenic risk score.
Copyright © 2023 Landoulsi, Pachchek, Bobbili, Pavelka, May, Krüger and the NCER-PD Consortium.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Anandhan A., Jacome M. S., Lei S., Hernandez-Franco P., Pappa A., Panayiotidis M. I., et al. (2017). Metabolic dysfunction in Parkinson’s disease: bioenergetics, redox homeostasis and central carbon metabolism. Brain Res. Bull. 133, 12–30. doi: 10.1016/j.brainresbull.2017.03.009, PMID: - DOI - PMC - PubMed
-
- Bandres-Ciga S., Saez-Atienzar S., Kim J. J., Makarious M. B., Faghri F., Diez-Fairen M., et al. (2020). Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol. 140, 341–358. doi: 10.1007/s00401-020-02181-3, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

