Molecular biology of canine parainfluenza virus V protein and its potential applications in tumor immunotherapy
- PMID: 38173672
- PMCID: PMC10761501
- DOI: 10.3389/fmicb.2023.1282112
Molecular biology of canine parainfluenza virus V protein and its potential applications in tumor immunotherapy
Abstract
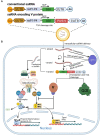
Canine parainfluenza virus (CPIV) is a zoonotic virus that is widely distributed and is the main pathogen causing canine infectious respiratory disease (CIRD), also known as "kennel cough," in dogs. The CPIV-V protein is the only nonstructural protein of the virus and plays an important role in multiple stages of the virus life cycle by inhibiting apoptosis, altering the host cell cycle and interfering with the interferon response. In addition, studies have shown that the V protein has potential applications in the field of immunotherapy in oncolytic virus therapy or self-amplifying RNA vaccines. In this review, the biosynthesis, structural characteristics and functions of the CPIV-V protein are reviewed with an emphasis on how it facilitates viral immune escape and its potential applications in the field of immunotherapy. Therefore, this review provides a scientific basis for research into the CPIV-V protein and its potential applications.
Keywords: V protein; canine parainfluenza virus; immune escape; molecular mechanism; structure; tumor immunotherapy; viral replication.
Copyright © 2023 Cheng, Zhang, Cai, Liu, Wen and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Detection of respiratory viruses and Bordetella bronchiseptica in dogs with acute respiratory tract infections.Vet J. 2014 Sep;201(3):365-9. doi: 10.1016/j.tvjl.2014.04.019. Epub 2014 May 6. Vet J. 2014. PMID: 24980809 Free PMC article.
-
Molecular detection and whole genome characterization of Canine Parainfluenza type 5 in Thailand.Sci Rep. 2021 Feb 16;11(1):3866. doi: 10.1038/s41598-021-83323-9. Sci Rep. 2021. PMID: 33594165 Free PMC article.
-
Molecular surveillance of traditional and emerging pathogens associated with canine infectious respiratory disease.Vet Microbiol. 2016 Aug 30;192:21-25. doi: 10.1016/j.vetmic.2016.06.009. Epub 2016 Jun 22. Vet Microbiol. 2016. PMID: 27527760 Free PMC article.
-
Aetiology of Canine Infectious Respiratory Disease Complex and Prevalence of its Pathogens in Europe.J Comp Pathol. 2020 Apr;176:86-108. doi: 10.1016/j.jcpa.2020.02.005. Epub 2020 Mar 17. J Comp Pathol. 2020. PMID: 32359641 Free PMC article. Review.
-
Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases.Rev Med Virol. 2018 Mar;28(2):e1965. doi: 10.1002/rmv.1965. Epub 2018 Jan 5. Rev Med Virol. 2018. PMID: 29316047 Free PMC article. Review.
References
-
- Andrus L., Marukian S., Jones C. T., Catanese M. T., Sheahan T. P., Schoggins J. W., et al. (2011). Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells. Hepatology 54, 1901–1912. doi: 10.1002/hep.24557, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

