Fibrous topology promoted pBMP2-activated matrix on titanium implants boost osseointegration
- PMID: 38173764
- PMCID: PMC10761207
- DOI: 10.1093/rb/rbad111
Fibrous topology promoted pBMP2-activated matrix on titanium implants boost osseointegration
Abstract
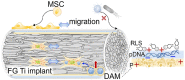
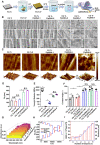
Titanium (Ti) implants have been extensively used after surgical operations. Its surface bioactivity is of importance to facilitate integration with surrounding bone tissue, and ultimately ensure stability and long-term functionality of the implant. The plasmid DNA-activated matrix (DAM) coating on the surface could benefit osseointegration but is still trapped by poor transfection for further application, especially on the bone marrow mesenchymal stem cells (BMSCs) in vivo practical conditions. Herein, we constructed a DAM on the surface of fibrous-grained titanium (FG Ti) composed of phase-transition lysozyme (P) as adhesive, cationic arginine-rich lipid (RLS) as the transfection agent and plasmid DNA (pDNA) for bone morphology protein 2 (BMP2) expression. The cationic lipid RLS improved up to 30-fold higher transfection than that of commercial reagents (Lipofectamine 2000 and polyethyleneimine) on MSC. And importantly, Ti surface topology not only promotes the DAM to achieve high transfection efficiency (∼75.7% positive cells) on MSC due to the favorable combination but also reserves its contact induction effect for osteoblasts. Upon further exploration, the fibrous topology on FG Ti could boost pDNA uptake for gene transfection, and cell migration in MSC through cytoskeleton remodeling and induce contact guidance for enhanced osteointegration. At the same time, the cationic RLS together with adhesive P were both antibacterial, showing up to 90% inhibition rate against Escherichia coli and Staphylococcus aureus with reduced adherent microorganisms and disrupted bacteria. Finally, the FG Ti-P/pBMP2 implant achieved accelerated bone healing capacities through highly efficient gene delivery, aligned surface topological structure and increased antimicrobial properties in a rat femoral condylar defect model.
Keywords: aligned surface topology; antibacterial activity; osseointegration; plasmid DNA-activated matrix.
© The Author(s) 2023. Published by Oxford University Press.
Figures





Similar articles
-
High Strength Titanium with Fibrous Grain for Advanced Bone Regeneration.Adv Sci (Weinh). 2023 Jun;10(16):e2207698. doi: 10.1002/advs.202207698. Epub 2023 Apr 7. Adv Sci (Weinh). 2023. PMID: 37029460 Free PMC article.
-
Transcript-Activated Coatings on Titanium Mediate Cellular Osteogenesis for Enhanced Osteointegration.Mol Pharm. 2021 Mar 1;18(3):1121-1137. doi: 10.1021/acs.molpharmaceut.0c01042. Epub 2021 Jan 25. Mol Pharm. 2021. PMID: 33492959 Free PMC article.
-
Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment.Acta Biomater. 2018 Oct 15;80:412-424. doi: 10.1016/j.actbio.2018.09.036. Epub 2018 Sep 25. Acta Biomater. 2018. PMID: 30266635
-
Gene-activated titanium implants for gene delivery to enhance osseointegration.Biomater Adv. 2022 Dec;143:213176. doi: 10.1016/j.bioadv.2022.213176. Epub 2022 Oct 27. Biomater Adv. 2022. PMID: 36327825 Review.
-
Peri-implant osteogenesis in health and osteoporosis.Micron. 2005;36(7-8):630-44. doi: 10.1016/j.micron.2005.07.008. Epub 2005 Sep 6. Micron. 2005. PMID: 16182543 Review.
Cited by
-
Effects of protein conformational transition accompanied with crosslinking density cues in silk fibroin hydrogels on the proliferation and chondrogenesis of encapsulated stem cells.Regen Biomater. 2025 Mar 20;12:rbaf019. doi: 10.1093/rb/rbaf019. eCollection 2025. Regen Biomater. 2025. PMID: 40290449 Free PMC article.
-
Y-shaped DNA as a dynamic self-assembly nanomaterial for phenotype-specific regulation of stem cell differentiation on the gene level.Regen Biomater. 2025 May 14;12:rbaf043. doi: 10.1093/rb/rbaf043. eCollection 2025. Regen Biomater. 2025. PMID: 40575763 Free PMC article.
-
Microfluidic 3D cell culture: potential application of collagen hydrogels with an optimal dose of bioactive glasses.Sci Rep. 2025 Jan 2;15(1):569. doi: 10.1038/s41598-024-84346-8. Sci Rep. 2025. PMID: 39747624 Free PMC article.
References
LinkOut - more resources
Full Text Sources

