Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: pitfall in managing immune-related adverse events
- PMID: 38173838
- PMCID: PMC10762640
- DOI: 10.3389/fonc.2023.1264281
Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: pitfall in managing immune-related adverse events
Abstract
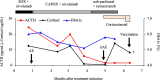
The occurrence of fulminant type 1 diabetes mellitus as an adverse event during cancer immunotherapy has been previously reported. However, little is known about the causal relationship between the coronavirus disease 2019 (COVID-19) vaccination and fulminant type 1 diabetes mellitus. A 60-year-old man with advanced gastric cancer, receiving S-1 + oxaliplatin and nivolumab therapy, followed by nab-paclitaxel + ramucirumab as a second-line treatment, with steroid supplementation for complications of hypopituitarism-induced hypoadrenocorticism, was administered a COVID-19 vaccine after three cycles of nab-paclitaxel + ramucirumab. Two days later, he developed severe malaise and anorexia, which required emergency admission to our hospital for suspected adrenal insufficiency. Despite increasing steroids, his general condition changed suddenly after 12 hours leading to his death. Histopathological analysis of autopsy samples revealed loss of the islets of Langerhans, indicating fulminant type 1 diabetes mellitus. We failed to recognize the onset of fulminant type 1 diabetes mellitus because its symptoms were similar to those of adrenal insufficiency. The number of reports on the onset of fulminant type 1 diabetes mellitus after COVID-19 vaccination has been increasing, and in this case, the onset occurred on the second day after COVID-19 vaccination, suggesting an association between vaccination and fulminant type 1 diabetes mellitus. Clinicians should be aware of the risk of fulminant type 1 diabetes mellitus, although rare, after COVID-19 vaccination.
Keywords: Autopsy; COVID-19 vaccination; Fulminant type 1 diabetes mellitus; gastric cancer; immune-related adverse event; nivolumab.
Copyright © 2023 Tanaka, Nagasu, Furuta, Gobaru, Suzuki, Shimotsuura, Akiba, Nomura, Fujita, Kawaguchi and Miwa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: A case report.J Diabetes Investig. 2022 Jul;13(7):1286-1289. doi: 10.1111/jdi.13771. Epub 2022 Feb 25. J Diabetes Investig. 2022. PMID: 35167186 Free PMC article.
-
Fulminant Type 1 Diabetes Mellitus Developed about Half a Year after Discontinuation of Immune Checkpoint Inhibitor Combination Therapy with Nivolumab and Ipilimumab: A Case Report.Tohoku J Exp Med. 2021 Aug;254(4):253-256. doi: 10.1620/tjem.254.253. Tohoku J Exp Med. 2021. PMID: 34373422
-
Painless Thyroiditis and Fulminant Type 1 Diabetes Mellitus in a Patient Treated with an Immune Checkpoint Inhibitor, Nivolumab.Tohoku J Exp Med. 2018 Jan;244(1):33-40. doi: 10.1620/tjem.244.33. Tohoku J Exp Med. 2018. PMID: 29343652
-
New-Onset Fulminant Type 1 Diabetes Following SARS-CoV-2 Protein Subunit Vaccine: A Case Report and Literature Review.J Korean Med Sci. 2023 Jun 19;38(24):e209. doi: 10.3346/jkms.2023.38.e209. J Korean Med Sci. 2023. PMID: 37337812 Free PMC article. Review.
-
COVID-19 Vaccination and Its Relation to New-Onset Diabetes: A Narrative Review.Cureus. 2023 Oct 15;15(10):e47056. doi: 10.7759/cureus.47056. eCollection 2023 Oct. Cureus. 2023. PMID: 38022276 Free PMC article. Review.
Cited by
-
Fulminant Type 1 Diabetes Mellitus With Concomitant Coxsackievirus B6 Antibody Elevation and RNA-Based COVID-19 Vaccination: A Case Report and Review of the Literature.Clin Case Rep. 2025 Apr 15;13(4):e70429. doi: 10.1002/ccr3.70429. eCollection 2025 Apr. Clin Case Rep. 2025. PMID: 40236311 Free PMC article.
-
Editorial: From bench to bedside in gastric cancer: diagnosis, prognosis, and treatment, volume II.Front Med (Lausanne). 2025 Mar 11;12:1562989. doi: 10.3389/fmed.2025.1562989. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40134913 Free PMC article. No abstract available.
-
Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism.Biomedicines. 2024 Jul 24;12(8):1650. doi: 10.3390/biomedicines12081650. Biomedicines. 2024. PMID: 39200115 Free PMC article. Review.
-
Case report: Strong GAD antibody positivity and type 1 diabetes-HLA-susceptible haplotype-DRB1*04:05-DQB1*04:01 in a Japanese patient with immune checkpoint inhibitor-induced type 1 diabetes.Front Endocrinol (Lausanne). 2024 May 22;15:1407192. doi: 10.3389/fendo.2024.1407192. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38841300 Free PMC article.
References
-
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. . First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2 - DOI - PMC - PubMed
-
- Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. . Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with her2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (Attraction-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6 - DOI - PubMed
-
- Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. . Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol (2017) 12(12):1798–805. doi: 10.1016/j.jtho.2017.08.022 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

