Lysosomal destabilization: A missing link between pathological calcification and osteoarthritis
- PMID: 38173842
- PMCID: PMC10761323
- DOI: 10.1016/j.bioactmat.2023.12.001
Lysosomal destabilization: A missing link between pathological calcification and osteoarthritis
Abstract
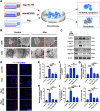
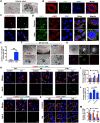
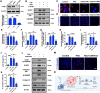
Calcification of cartilage by hydroxyapatite is a hallmark of osteoarthritis and its deposition strongly correlates with the severity of osteoarthritis. However, no effective strategies are available to date on the prevention of hydroxyapatite deposition within the osteoarthritic cartilage and its role in the pathogenesis of this degenerative condition is still controversial. Therefore, the present work aims at uncovering the pathogenic mechanism of intra-cartilaginous hydroxyapatite in osteoarthritis and developing feasible strategies to counter its detrimental effects. With the use of in vitro and in vivo models of osteoarthritis, hydroxyapatite crystallites deposited in the cartilage are found to be phagocytized by resident chondrocytes and processed by the lysosomes of those cells. This results in lysosomal membrane permeabilization (LMP) and release of cathepsin B (CTSB) into the cytosol. The cytosolic CTSB, in turn, activates NOD-like receptor protein-3 (NLRP3) inflammasomes and subsequently instigates chondrocyte pyroptosis. Inhibition of LMP and CTSB in vivo are effective in managing the progression of osteoarthritis. The present work provides a conceptual therapeutic solution for the prevention of osteoarthritis via alleviation of lysosomal destabilization.
Keywords: Chondrocyte pyroptosis; Hydroxyapatite; Lysosomal destabilization; Osteoarthritis; Pathological calcification.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Apigenin Alleviated High-Fat-Diet-Induced Hepatic Pyroptosis by Mitophagy-ROS-CTSB-NLRP3 Pathway in Mice and AML12 Cells.J Agric Food Chem. 2023 May 10;71(18):7032-7045. doi: 10.1021/acs.jafc.2c07581. Epub 2023 May 4. J Agric Food Chem. 2023. PMID: 37141464
-
27-Hydroxycholesterol Contributes to Lysosomal Membrane Permeabilization-Mediated Pyroptosis in Co-cultured SH-SY5Y Cells and C6 Cells.Front Mol Neurosci. 2019 Mar 1;12:14. doi: 10.3389/fnmol.2019.00014. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30881285 Free PMC article.
-
Elaidic acid induced hepatocyte pyroptosis via autophagy-CTSB-NLRP3 pathway.Food Chem Toxicol. 2023 Nov;181:114060. doi: 10.1016/j.fct.2023.114060. Epub 2023 Sep 23. Food Chem Toxicol. 2023. PMID: 37748573
-
Counterpoint: Hydroxyapatite crystal deposition is not intimately involved in the pathogenesis and progression of human osteoarthritis.Curr Rheumatol Rep. 2009 Apr;11(2):148-53. doi: 10.1007/s11926-009-0021-5. Curr Rheumatol Rep. 2009. PMID: 19296888 Review.
-
Pathological calcification in osteoarthritis: an outcome or a disease initiator?Biol Rev Camb Philos Soc. 2020 Aug;95(4):960-985. doi: 10.1111/brv.12595. Epub 2020 Mar 24. Biol Rev Camb Philos Soc. 2020. PMID: 32207559 Review.
Cited by
-
Genetic insights into serum cathepsins as diagnostic and therapeutic targets in knee and hip osteoarthritis.Sci Rep. 2024 Jul 30;14(1):17553. doi: 10.1038/s41598-024-68718-8. Sci Rep. 2024. PMID: 39080459 Free PMC article.
-
M6A Demethyltransferase FTO Attenuates Meniscus Degeneration and Osteoarthritis via Orchestrating Autophagy and Energetic Metabolism.Adv Sci (Weinh). 2025 Mar;12(9):e2412379. doi: 10.1002/advs.202412379. Epub 2025 Jan 13. Adv Sci (Weinh). 2025. PMID: 39804978 Free PMC article.
-
Dental follicle stem cell-derived small extracellular vesicles ameliorate pulpitis by reprogramming macrophage metabolism.Bioact Mater. 2025 May 12;51:179-196. doi: 10.1016/j.bioactmat.2025.04.034. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40475085 Free PMC article.
-
Programmed enhancement of endogenous iron-mediated lysosomal membrane permeabilization for tumor ferroptosis/pyroptosis dual-induction.Nat Commun. 2025 Mar 28;16(1):3017. doi: 10.1038/s41467-025-58124-7. Nat Commun. 2025. PMID: 40148335 Free PMC article.
-
Neurovascularization inhibiting dual responsive hydrogel for alleviating the progression of osteoarthritis.Nat Commun. 2025 Feb 6;16(1):1390. doi: 10.1038/s41467-025-56727-8. Nat Commun. 2025. PMID: 39910066 Free PMC article.
References
-
- Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):33–42. - PubMed
-
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. - PubMed
-
- Dai B., Zhu Y., Li X., Liang Z., Xu S., Zhang S., Zhang Z., Bai S., Tong W., Cao M., Li Y., Zhu X., Liu W., Zhang Y., Chang L., Yung P.S., Ki-Wai Ho K., Xu J., Ngai T., Qin L. Blockage of osteopontin-integrin beta3 signaling in infrapatellar fat pad attenuates osteoarthritis in mice. Adv. Sci. 2023;10(22) - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

