Mechanical properties, and in vitro biocompatibility assessment of biomimetic dual layered keratin/ hydroxyapatite scaffolds
- PMID: 38173873
- PMCID: PMC10764155
- DOI: 10.3389/fbioe.2023.1304147
Mechanical properties, and in vitro biocompatibility assessment of biomimetic dual layered keratin/ hydroxyapatite scaffolds
Abstract
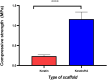
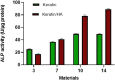
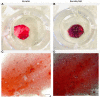
A novel biomimetic dual layered keratin/hydroxyapatite (keratin/HA) scaffold was designed using iterative freeze-drying technique. The prepared scaffolds were studied using several analytical techniques to better understand the biological, structural, and mechanical properties. The developed multilayered, interconnected, porous keratin scaffold with different hydroxyapatite (HA) content in the outer and inner layer, mimics the inherent gradient structure of alveolar bone. SEM studies showed an interconnected porous architecture of the prepared scaffolds with seamless integration between the upper and lower layers. The incorporation of HA improved the mechanical properties keratin/HA scaffolds. The keratin/HA scaffolds exhibited superior mechanical properties in terms of Young's modulus and compressive strength in comparison to pure keratin scaffolds. The biocompatibility studies suggested that both keratin and keratin/HA scaffolds were cyto-compatible, in terms of cell proliferation. Furthermore, it showed that both the tested materials can served as an ideal substrate for the differentiation of Saos-2 cells, leading to mineralization of the extracellular matrix. In summary, ionic liquid based green technique was employed for keratin extraction to fabricate keratin/HA scaffolds and our detailed in vitro investigations suggest the great potential for these composite scaffolds for bone tissue engineering in future.
Keywords: alveolar bone; biomimetic; bone tissue engineering; dental implants; keratin.
Copyright © 2023 Feroz, Muhammad, Ullah, Nishan, Cathro and Dias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Hydroxypropylmethyl cellulose (HPMC) crosslinked keratin/hydroxyapatite (HA) scaffold fabrication, characterization and in vitro biocompatibility assessment as a bone graft for alveolar bone regeneration.Heliyon. 2021 Oct 29;7(11):e08294. doi: 10.1016/j.heliyon.2021.e08294. eCollection 2021 Nov. Heliyon. 2021. PMID: 34765797 Free PMC article.
-
Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109960. doi: 10.1016/j.msec.2019.109960. Epub 2019 Jul 6. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31500051
-
[Preparation and in vitro evaluation of tissue engineered osteochondral integration of multi-layered scaffold].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Apr 15;32(4):434-440. doi: 10.7507/1002-1892.201712038. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806301 Free PMC article. Chinese.
-
Alginate-hydroxyapatite scaffolds: A comprehensive characterization study.J Oral Biol Craniofac Res. 2025 May-Jun;15(3):555-562. doi: 10.1016/j.jobcr.2025.03.010. Epub 2025 Mar 24. J Oral Biol Craniofac Res. 2025. PMID: 40212102 Free PMC article.
-
Unlocking the Potential of Keratin: A Comprehensive Exploration from Extraction and Structural Properties to Cross-Disciplinary Applications.J Agric Food Chem. 2025 Jan 15;73(2):1014-1037. doi: 10.1021/acs.jafc.4c07102. Epub 2024 Dec 16. J Agric Food Chem. 2025. PMID: 39681472 Review.
Cited by
-
Laboratory performance of novel injectable keratin nanoparticle from poultry feathers loaded-alginate composite hydrogel as a pulpotomy filling biomaterial for dentin regeneration.BMC Oral Health. 2025 Aug 26;25(1):1377. doi: 10.1186/s12903-025-06535-9. BMC Oral Health. 2025. PMID: 40859215 Free PMC article.
-
Injectable bioactive scaffold able to stimulate oral bone regeneration on demand.J Mater Sci Mater Med. 2025 Apr 8;36(1):31. doi: 10.1007/s10856-025-06879-2. J Mater Sci Mater Med. 2025. PMID: 40198381 Free PMC article.
References
-
- Al-Munajjed A. A., Plunkett N. A., Gleeson J. P., Weber T., Jungreuthmayer C., Levingstone T., et al. (2009). Development of a biomimetic collagen‐hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J. Biomed. Mater. Res. Part B Appl. Biomaterials An Official J. Soc. Biomaterials 90 (2), 584–591. 10.1002/jbm.b.31320 - DOI - PubMed
-
- Bavaresco B., Comín R., Salvatierra N. A., Cid M. P. (2020). Three-dimensional printing of collagen and hyaluronic acid scaffolds with dehydrothermal treatment crosslinking. Compos. Commun. 19, 1–5. 10.1016/j.coco.2020.02.001 - DOI
-
- Chen C.-S., Chang J.-H., Srimaneepong V., Wen J.-Y., Tung O.-H., Yang C.-H., et al. (2020). Improving the in vitro cell differentiation and in vivo osseointegration of titanium dental implant through oxygen plasma immersion ion implantation treatment. Surf. Coatings Technol. 399, 126125. 10.1016/j.surfcoat.2020.126125 - DOI
-
- Chen L., Wu Z., Zhou Y., Li L., Wang Y., Wang Z., et al. (2017). Biomimetic porous collagen/hydroxyapatite scaffold for bone tissue engineering. J. Appl. Polym. Sci. 134 (37), 45271. 10.1002/app.45271 - DOI
LinkOut - more resources
Full Text Sources

