Synthesis of triazole bridged N-glycosides of pyrazolo[1,5- a]pyrimidinones as anticancer agents and their in silico docking studies
- PMID: 38174229
- PMCID: PMC10762718
- DOI: 10.1039/d3ra06993a
Synthesis of triazole bridged N-glycosides of pyrazolo[1,5- a]pyrimidinones as anticancer agents and their in silico docking studies
Abstract
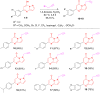
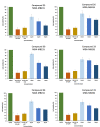
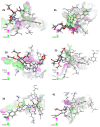
In the pursuit of novel therapeutic agents, we present a comprehensive study on the design, synthesis, and evaluation of a diverse library of triazole bridged N-glycosides of pyrazolo[1,5-a]pyrimidinones, employing a microwave-assisted synthetic approach via 'click chemistry'. This methodology offers efficient and accelerated access to the glycohybrids, showcasing improved reaction conditions that yield high-quality products. In this research endeavor, we have successfully synthesized a series of twenty-seven triazole bridged N-glycosides of pyrazolo[1,5-a]pyrimidinones. Our investigation extends beyond synthetic endeavors to explore the potential therapeutic relevance of these compounds. We subjected them to rigorous in vitro screening against prominent breast cancer cell lines MCF-7, MDA-MB231, and MDA-MB453. Among the library of compounds synthesized, (2S,3S,4R,5S,6S)-2-(acetoxymethyl)-6-(4-((5-(4-methoxyphenyl)-7-oxopyrazolo[1,5-a]pyrimidin-1(7H)-yl)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate emerged as a potent compound, exhibiting remarkable anti-cancer activity with an IC50 value of 27.66 μM against the MDA-MB231 cell line. Additionally, (2S,3R,4R,5S,6S)-2-(acetoxymethyl)-6-(4-((7-oxo-5-(4-(trifluoromethyl)phenyl)pyrazolo[1,5-a]pyrimidin-1(7H)-yl)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate displayed notable inhibitory potential against the MCF-7 cell line, with an IC50 value of 4.93 μM. Furthermore, in silico docking analysis was performed to validate our experimental findings. These findings underscore the promise of our triazole bridged N-glycosides of pyrazolo[1,5-a]pyrimidinones as potential anti-cancer agents. This research not only enriches the field of glycohybrid synthesis but also contributes valuable insights into the development of novel anti-cancer therapeutics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Copper-catalyzed synthesis of pyrazolo[1,5-a]pyrimidine based triazole-linked glycohybrids: mechanistic insights and bio-applications.Sci Rep. 2024 Jan 4;14(1):529. doi: 10.1038/s41598-023-50202-4. Sci Rep. 2024. PMID: 38177184 Free PMC article.
-
Efficient synthesis of indole-chalcones based glycohybrids and their anticancer activity.Bioorg Med Chem. 2024 Jul 15;109:117778. doi: 10.1016/j.bmc.2024.117778. Epub 2024 May 30. Bioorg Med Chem. 2024. PMID: 38870714
-
Molecular Design, Synthesis and Anti-cancer Activity of Novel Pyrazolo[3,4-b]pyridine-based Glycohybrid Molecules.Bioorg Chem. 2025 Mar;156:108161. doi: 10.1016/j.bioorg.2025.108161. Epub 2025 Jan 17. Bioorg Chem. 2025. PMID: 39848166
-
Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation.Bioorg Chem. 2022 Jul;124:105816. doi: 10.1016/j.bioorg.2022.105816. Epub 2022 Apr 16. Bioorg Chem. 2022. PMID: 35489270 Review.
-
Development of allosteric modulators of GPCRs for treatment of CNS disorders.Neurobiol Dis. 2014 Jan;61:55-71. doi: 10.1016/j.nbd.2013.09.013. Epub 2013 Sep 27. Neurobiol Dis. 2014. PMID: 24076101 Free PMC article. Review.
References
-
- Walsh C. T. Tetrahedron Lett. 2015;56:3075–3081.
-
- Sharma P. K. Amin A. Kumar M. Open J. Med. Chem. 2020;14:49–64.
-
- Burgart Y. V. Elkina N. A. Shchegolkov E. V. Krasnykh O. P. Maslova V. V. Triandafilova G. A. Solodnikov S. Y. Makhaeva G. F. Serebryakova O. G. Rudakova E. V. Saloutin V. I. Chem. Heterocycl. Compd. 2020;56:199–207.
-
- Markwalder J. A. Arnone M. R. Benfield P. A. Boisclair M. Burton C. R. Chang C.-H. Cox S. S. Czerniak P. M. Dean C. L. Doleniak D. Grafstrom R. Harrison B. A. Kaltenbach R. F. Nugiel D. A. Rossi K. A. Sherk S. R. Sisk L. M. Stouten P. Trainor G. L. Worland P. Seitz S. P. J. Med. Chem. 2004;47:5894–5911. - PubMed
LinkOut - more resources
Full Text Sources

