Photo-responsive nanoporous liquid crystal polymer films for selective dye adsorption
- PMID: 38174275
- PMCID: PMC10759169
- DOI: 10.1039/d3ra06791b
Photo-responsive nanoporous liquid crystal polymer films for selective dye adsorption
Abstract
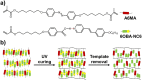
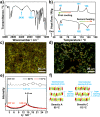
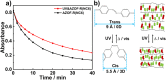
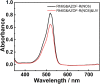
Photo-responsive nanoporous polymer films (AZOF-R(NC6)) have been developed by a template method based on a hydrogen-bonding supramolecular liquid crystal (LC) and a light-sensitive azobenzene LC crosslinker in this work. Anionic nanopores were obtained after the removal of template NC6 using KOH solution. The AZOF-R(NC6) demonstrates charge-selective dye adsorption and the maximum adsorption capacity for Rh6G is 504.6 mg g-1. The AZOF-R(NC6) film without UV light treatment shows a 32% higher adsorption capacity for Rh6G than the AZOF-R(NC6) film treated with UV light within the initial 10 min. In addition, UV light can trigger the release of the adsorbed dye from the polymer film due to the pore size change arising from the trans-cis isomerization. Besides, the used polymer film can be effectively regenerated using a HCl solution. Such functional polymer films with highly ordered nanopores and photo-responsive properties hold great promise in selective adsorption and mass separations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
NIR-Vis-UV Light-Responsive Actuator Films of Polymer-Dispersed Liquid Crystal/Graphene Oxide Nanocomposites.ACS Appl Mater Interfaces. 2015 Dec 16;7(49):27494-501. doi: 10.1021/acsami.5b09676. Epub 2015 Dec 1. ACS Appl Mater Interfaces. 2015. PMID: 26592303
-
Surface-modified silica colloidal crystals: nanoporous films and membranes with controlled ionic and molecular transport.Acc Chem Res. 2014 Feb 18;47(2):440-9. doi: 10.1021/ar400157w. Epub 2014 Jan 7. Acc Chem Res. 2014. PMID: 24397245
-
Homeotropic Self-Alignment of Discotic Liquid Crystals for Nanoporous Polymer Films.ACS Nano. 2018 Jul 24;12(7):6714-6724. doi: 10.1021/acsnano.8b01822. Epub 2018 Jul 10. ACS Nano. 2018. PMID: 29975513 Free PMC article.
-
Motion of Adsorbed Nano-Particles on Azobenzene Containing Polymer Films.Molecules. 2016 Dec 3;21(12):1663. doi: 10.3390/molecules21121663. Molecules. 2016. PMID: 27918473 Free PMC article. Review.
-
Meso- and microscopic motions in photoresponsive liquid crystalline polymer films.Macromol Rapid Commun. 2014 Feb;35(3):271-90. doi: 10.1002/marc.201300763. Epub 2013 Dec 17. Macromol Rapid Commun. 2014. PMID: 24343758 Review.
References
-
- Jackson E. A. Hillmyer M. A. ACS Nano. 2010;4:3548. - PubMed
-
- Lee J. H. Han K. S. Lee J. S. Lee A. S. Park S. K. Hong S. Y. Lee J. C. Mueller K. T. Hong S. M. Koo C. M. Adv. Mater. 2016;28:9301. - PubMed
-
- Wu D. Xu F. Sun B. Fu R. He H. Matyjaszewski K. Chem. Rev. 2012;112:3959. - PubMed
-
- Schenning A. P. H. J. Gonzalez-Lemus Y. C. Shishmanova I. K. Broer D. J. Liq. Cryst. 2011;38:1627.
LinkOut - more resources
Full Text Sources

