Pericyte loss initiates microvascular dysfunction in the development of diastolic dysfunction
- PMID: 38174347
- PMCID: PMC10763525
- DOI: 10.1093/ehjopen/oead129
Pericyte loss initiates microvascular dysfunction in the development of diastolic dysfunction
Abstract
Aims: Microvascular dysfunction has been proposed to drive heart failure with preserved ejection fraction (HFpEF), but the initiating molecular and cellular events are largely unknown. Our objective was to determine when microvascular alterations in HFpEF begin, how they contribute to disease progression, and how pericyte dysfunction plays a role herein.
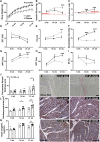
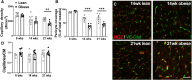
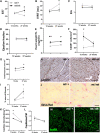
Methods and results: Microvascular dysfunction, characterized by inflammatory activation, loss of junctional barrier function, and altered pericyte-endothelial crosstalk, was assessed with respect to the development of cardiac dysfunction, in the Zucker fatty and spontaneously hypertensive (ZSF1) obese rat model of HFpEF at three time points: 6, 14, and 21 weeks of age. Pericyte loss was the earliest and strongest microvascular change, occurring before prominent echocardiographic signs of diastolic dysfunction were present. Pericytes were shown to be less proliferative and had a disrupted morphology at 14 weeks in the obese ZSF1 animals, who also exhibited an increased capillary luminal diameter and disrupted endothelial junctions. Microvascular dysfunction was also studied in a mouse model of chronic reduction in capillary pericyte coverage (PDGF-Bret/ret), which spontaneously developed many aspects of diastolic dysfunction. Pericytes exposed to oxidative stress in vitro showed downregulation of cell cycle-associated pathways and induced a pro-inflammatory state in endothelial cells upon co-culture.
Conclusion: We propose pericytes are important for maintaining endothelial cell function, where loss of pericytes enhances the reactivity of endothelial cells to inflammatory signals and promotes microvascular dysfunction, thereby accelerating the development of HFpEF.
Keywords: HFpEF; Metabolic comorbidities; Microvascular dysfunction; Pericytes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None.
Figures

References
-
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. - PubMed
-
- Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, Lang NN, Jhund PS, Campbell RT, McMurray JJV, Petrie MC. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021;6:1130–1143. - PMC - PubMed
-
- Ahmad A, Corban MT, Toya T, Verbrugge FH, Sara JD, Lerman LO, Borlaug BA, Lerman A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:765–772. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

