A transdiagnostic and translational framework for delineating the neuronal mechanisms of compulsive exercise in anorexia nervosa
- PMID: 38174745
- PMCID: PMC11222308
- DOI: 10.1002/eat.24130
A transdiagnostic and translational framework for delineating the neuronal mechanisms of compulsive exercise in anorexia nervosa
Abstract
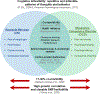
Objective: The development of novel treatments for anorexia nervosa (AN) requires a detailed understanding of the biological underpinnings of specific, commonly occurring symptoms, including compulsive exercise. There is considerable bio-behavioral overlap between AN and obsessive-compulsive disorder (OCD), therefore it is plausible that similar mechanisms underlie compulsive behavior in both populations. While the association between these conditions is widely acknowledged, defining the shared mechanisms for compulsive behavior in AN and OCD requires a novel approach.
Methods: We present an argument that a better understanding of the neurobiological mechanisms that underpin compulsive exercise in AN can be achieved in two critical ways. First, by applying a framework of the neuronal control of OCD to exercise behavior in AN, and second, by taking better advantage of the activity-based anorexia (ABA) rodent model to directly test this framework in the context of feeding pathology.
Results: A cross-disciplinary approach that spans preclinical, neuroimaging, and clinical research as well as compulsive neurocircuitry and behavior can advance our understanding of when, why, and how compulsive exercise develops in the context of AN and provide targets for novel treatment strategies.
Discussion: In this article, we (i) link the expression of compulsive behavior in AN and OCD via a transition between goal-directed and habitual behavior, (ii) present disrupted cortico-striatal circuitry as a key substrate for the development of compulsive behavior in both conditions, and (iii) highlight the utility of the ABA rodent model to better understand the mechanisms of compulsive behavior relevant to AN.
Public significance: Individuals with AN who exercise compulsively are at risk of worse health outcomes and have poorer responses to standard treatments. However, when, why, and how compulsive exercise develops in AN remains inadequately understood. Identifying whether the neural circuitry underlying compulsive behavior in OCD also controls hyperactivity in the activity-based anorexia model will aid in the development of novel eating disorder treatment strategies for this high-risk population.
Keywords: activity‐based anorexia; anorexia nervosa; compulsive exercise; cortico‐striatal circuits; obsessive‐compulsive disorder.
© 2024 The Authors. International Journal of Eating Disorders published by Wiley Periodicals LLC.
Figures

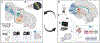
References
-
- Balleine BW (2011). Sensation, Incentive Learning, and the Motivational Control of Goal-Directed Action. In Gottfried JA (Ed.), Neurobiology of Sensation and Reward. https://www.ncbi.nlm.nih.gov/pubmed/22593900 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

