Unveiling the role of host kinases at different steps of influenza A virus life cycle
- PMID: 38174932
- PMCID: PMC10805039
- DOI: 10.1128/jvi.01192-23
Unveiling the role of host kinases at different steps of influenza A virus life cycle
Abstract
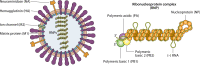
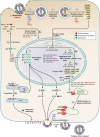
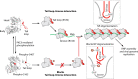
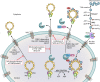
Influenza viruses remain a major public health concern causing contagious respiratory illnesses that result in around 290,000-650,000 global deaths every year. Their ability to constantly evolve through antigenic shifts and drifts leads to the emergence of newer strains and resistance to existing drugs and vaccines. To combat this, there is a critical need for novel antiviral drugs through the introduction of host-targeted therapeutics. Influenza viruses encode only 14 gene products that get extensively modified through phosphorylation by a diverse array of host kinases. Reversible phosphorylation at serine, threonine, or tyrosine residues dynamically regulates the structure, function, and subcellular localization of viral proteins at different stages of their life cycle. In addition, kinases influence a plethora of signaling pathways that also regulate virus propagation by modulating the host cell environment thus establishing a critical virus-host relationship that is indispensable for executing successful infection. This dependence on host kinases opens up exciting possibilities for developing kinase inhibitors as next-generation anti-influenza therapy. To fully capitalize on this potential, extensive mapping of the influenza virus-host kinase interaction network is essential. The key focus of this review is to outline the molecular mechanisms by which host kinases regulate different steps of the influenza A virus life cycle, starting from attachment-entry to assembly-budding. By assessing the contributions of different host kinases and their specific phosphorylation events during the virus life cycle, we aim to develop a holistic overview of the virus-host kinase interaction network that may shed light on potential targets for novel antiviral interventions.
Keywords: ERK; MAP kinase; PKC; RNP assembly; RNP export; host kinases; influenza; viral transcription and replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Influenza Virus Infections and Cellular Kinases.Viruses. 2019 Feb 20;11(2):171. doi: 10.3390/v11020171. Viruses. 2019. PMID: 30791550 Free PMC article. Review.
-
Phosphorylation of JIP4 at S730 Presents Antiviral Properties against Influenza A Virus Infection.J Virol. 2021 Sep 27;95(20):e0067221. doi: 10.1128/JVI.00672-21. Epub 2021 Jul 28. J Virol. 2021. PMID: 34319782 Free PMC article.
-
The Nucleolar Protein LYAR Facilitates Ribonucleoprotein Assembly of Influenza A Virus.J Virol. 2018 Nov 12;92(23):e01042-18. doi: 10.1128/JVI.01042-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209172 Free PMC article.
-
Protein Tyrosine Phosphatase SHP2 Suppresses Host Innate Immunity against Influenza A Virus by Regulating EGFR-Mediated Signaling.J Virol. 2021 Feb 24;95(6):e02001-20. doi: 10.1128/JVI.02001-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361428 Free PMC article.
-
Interplay between influenza A virus and host factors: targets for antiviral intervention.Arch Virol. 2015 Aug;160(8):1877-91. doi: 10.1007/s00705-015-2452-9. Epub 2015 May 29. Arch Virol. 2015. PMID: 26016443 Review.
Cited by
-
Screening kinase inhibitors identifies MELK as a prime target against influenza virus infections through inhibition of viral mRNA splicing.Front Microbiol. 2025 Jun 5;16:1600935. doi: 10.3389/fmicb.2025.1600935. eCollection 2025. Front Microbiol. 2025. PMID: 40539108 Free PMC article.
-
Architectural organization and in situ fusion protein structure of lymphocytic choriomeningitis virus.J Virol. 2024 Oct 22;98(10):e0064024. doi: 10.1128/jvi.00640-24. Epub 2024 Sep 27. J Virol. 2024. PMID: 39329471 Free PMC article.
-
LDC000067, a CDK9 inhibitor, unveils promising results in suppressing influenza virus infections in vitro and in vivo.Antimicrob Agents Chemother. 2025 Jan 31;69(1):e0117224. doi: 10.1128/aac.01172-24. Epub 2024 Nov 20. Antimicrob Agents Chemother. 2025. PMID: 39565117 Free PMC article.
-
Targeting Non-Eosinophilic Immunological Pathways in COPD and AECOPD: Current Insights and Therapeutic Strategies.Int J Chron Obstruct Pulmon Dis. 2025 Mar 5;20:511-532. doi: 10.2147/COPD.S506616. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40066199 Free PMC article. Review.
-
Strategies and efforts in circumventing the emergence of antiviral resistance against conventional antivirals.NPJ Antimicrob Resist. 2025 Jun 9;3(1):54. doi: 10.1038/s44259-025-00125-z. NPJ Antimicrob Resist. 2025. PMID: 40490516 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

