Real-world outcomes of nivolumab plus ipilimumab and pembrolizumab with platinum-based chemotherapy in advanced non-small cell lung cancer: a multicenter retrospective comparative study
- PMID: 38175294
- PMCID: PMC10766714
- DOI: 10.1007/s00262-023-03583-4
Real-world outcomes of nivolumab plus ipilimumab and pembrolizumab with platinum-based chemotherapy in advanced non-small cell lung cancer: a multicenter retrospective comparative study
Abstract
Introduction: Nivolumab plus ipilimumab with chemotherapy (NICT) and pembrolizumab with chemotherapy (PCT) are commonly used in patients with advanced non-small cell lung cancer (NSCLC). Compared with immune checkpoint inhibitor (ICI) monotherapy, ICI combination therapy can increase immune-related toxicity instead of prolonging survival. This study aimed to compare the efficacy and safety of NICT and PCT to decide on the favorable treatment.
Methods: We conducted a multi-center retrospective cohort study on patients who underwent NICT or PCT between December 2018 and May 2022. Propensity score matching (PSM) was performed with the variables age, sex, smoking status, performance status, stage, histology, and programmed cell death ligand-1 (PD-L1). The Kaplan-Meier method was used to compare survival for the matched patients.
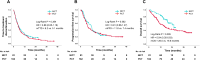
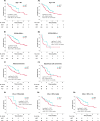
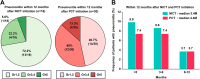
Results: Six hundred consecutive patients were included. After PSM, 81 and 162 patients were enrolled in the NICT and PCT groups, respectively. The baseline characteristics were well-balanced. The median progression-free survival was equivalent (11.6 vs. 7.4 months; P = 0.582); however, the median overall survival (OS) was significantly longer in the NICT group than in the PCT group (26.0 vs. 16.8 months; P = 0.005). Furthermore, OS was better in PD-L1-negative patients who underwent NICT than in those who underwent PCT (26.0 vs. 16.8 months; P = 0.045). Safety profiles did not differ significantly in terms of severe adverse event and treatment-related death rates (P = 0.560, and 0.722, respectively).
Conclusions: Real-world data suggests that NICT could be a favorable treatment option compared with PCT for patients with advanced NSCLC. Further follow-up is needed to determine the long-term prognostic benefit.
Keywords: Ipilimumab; Nivolumab; Non-small-cell Lung cancer; Pembrolizumab; Propensity score; Real-world.
© 2023. The Author(s).
Conflict of interest statement
Dr. A. Tamiya reports receiving grants from AstraZeneca, BeiGene, and Daiichi-Sankyo; and honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Pfizer, Amgen, Kyowa Kirin, Merck BioFarma, Novartis, Ono Pharmacaceutical, Chugai Pharmacaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Merck Sharp & Dohme, Takeda Pharmaceutical, Nihon-Kayaku, and Thermo Fischer Scientific outside of the submitted work. Dr. M. Tamiya reports receiving grants from Ono Pharmaceutical, Japan, Bristol-Myers Squibb, and Boehringer Ingelheim; and honoraria for lectures from Taiho Pharmaceutical, Japan, Eli Lilly, Asahi Kasei Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, and Bristol-Myers Squibb outside of the submitted work. Dr. Nishino reports receiving grants from Ono Pharmaceutical, Taiho Pharmaceutical, Merck Sharp & Dohme, AbbVie, Daiichi-Sankyo, Amgen, Eisai, Sanofi, Janssen Pharmaceutical, Novartis, Pfizer, Eli Lilly Japan, Merck Biopharma, Takeda Pharmaceutical, Chugai Pharmaceutical, Merus; and honoraria for lectures from AstraZeneca, Chugai pharmaceutical, Nippon Boehringer Ingelheim, Eli Lilly Japan, Roche Diagnostics, Novartis, Pfizer, Merk, Janssen Pharmaceutical, Bristol-Myers Squibb, Nippon Kayaku outside of the submitted work. Dr. Mori. reports receiving grants from Ono Pharmaceutical, Merck Sharp & Dohme, Delt-Fly Pharma, Chugai Pharmaceutical; and honoraria for lectures from AstraZeneca, Chugai pharmaceutical, Nippon Boehringer Ingelheim, Eli Lilly Japan, Novartis, Pfizer, Merck Sharp & Dohme, Bristol-Myers Squibb, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda Pharmaceutical, Nippon Kayaku, Kyowa Kirin, Daiichi-Sankyo, Otsuka Pharmaceutical, Shionogi Pharmaceutical outside of the submitted work. Dr. Kijima. reports receiving honoraria for lectures from Chugai pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical outside of the submitted work. The other authors declare no conflicts of interest.
Figures
References
-
- Paz-Ares L, Ciuleanu T, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell Lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. - DOI - PubMed
-
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell Lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

