Cortical matrix remodeling as a hallmark of relapsing-remitting neuroinflammation in MR elastography and quantitative MRI
- PMID: 38175305
- PMCID: PMC10766667
- DOI: 10.1007/s00401-023-02658-x
Cortical matrix remodeling as a hallmark of relapsing-remitting neuroinflammation in MR elastography and quantitative MRI
Abstract
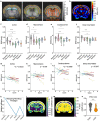
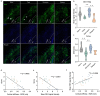
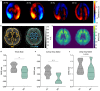
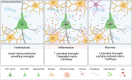
Multiple sclerosis (MS) is a chronic neuroinflammatory disease that involves both white and gray matter. Although gray matter damage is a major contributor to disability in MS patients, conventional clinical magnetic resonance imaging (MRI) fails to accurately detect gray matter pathology and establish a clear correlation with clinical symptoms. Using magnetic resonance elastography (MRE), we previously reported global brain softening in MS and experimental autoimmune encephalomyelitis (EAE). However, it needs to be established if changes of the spatiotemporal patterns of brain tissue mechanics constitute a marker of neuroinflammation. Here, we use advanced multifrequency MRE with tomoelastography postprocessing to investigate longitudinal and regional inflammation-induced tissue changes in EAE and in a small group of MS patients. Surprisingly, we found reversible softening in synchrony with the EAE disease course predominantly in the cortex of the mouse brain. This cortical softening was associated neither with a shift of tissue water compartments as quantified by T2-mapping and diffusion-weighted MRI, nor with leukocyte infiltration as seen by histopathology. Instead, cortical softening correlated with transient structural remodeling of perineuronal nets (PNNs), which involved abnormal chondroitin sulfate expression and microgliosis. These mechanisms also appear to be critical in humans with MS, where tomoelastography for the first time demonstrated marked cortical softening. Taken together, our study shows that neuroinflammation (i) critically affects the integrity of PNNs in cortical brain tissue, in a reversible process that correlates with disease disability in EAE, (ii) reduces the mechanical integrity of brain tissue rather than leading to water accumulation, and (iii) shows similar spatial patterns in humans and mice. These results raise the prospect of leveraging MRE and quantitative MRI for MS staging and monitoring treatment in affected patients.
Keywords: Cerebral cortex; Experimental autoimmune encephalomyelitis; Magnetic resonance elastography; Multiple sclerosis; Perineuronal nets; Tomoelastography.
© 2024. The Author(s).
Figures



Similar articles
-
Contribution of Tissue Inflammation and Blood-Brain Barrier Disruption to Brain Softening in a Mouse Model of Multiple Sclerosis.Front Neurosci. 2021 Aug 23;15:701308. doi: 10.3389/fnins.2021.701308. eCollection 2021. Front Neurosci. 2021. PMID: 34497486 Free PMC article.
-
Functional Magnetic Resonance Imaging of Rats with Experimental Autoimmune Encephalomyelitis Reveals Brain Cortex Remodeling.J Neurosci. 2015 Jul 8;35(27):10088-100. doi: 10.1523/JNEUROSCI.0540-15.2015. J Neurosci. 2015. PMID: 26157006 Free PMC article.
-
Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis.Neuroimage Clin. 2012 Sep 12;1(1):81-90. doi: 10.1016/j.nicl.2012.09.003. eCollection 2012. Neuroimage Clin. 2012. PMID: 24179740 Free PMC article.
-
Diffusion-weighted MR of the brain: methodology and clinical application.Radiol Med. 2005 Mar;109(3):155-97. Radiol Med. 2005. PMID: 15775887 Review. English, Italian.
-
Magnetic resonance techniques to quantify tissue damage, tissue repair, and functional cortical reorganization in multiple sclerosis.Prog Brain Res. 2009;175:465-82. doi: 10.1016/S0079-6123(09)17531-3. Prog Brain Res. 2009. PMID: 19660674 Review.
Cited by
-
Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues.Cells. 2024 Oct 1;13(19):1638. doi: 10.3390/cells13191638. Cells. 2024. PMID: 39404401 Free PMC article. Review.
-
The Networking Brain: How Extracellular Matrix, Cellular Networks, and Vasculature Shape the In Vivo Mechanical Properties of the Brain.Adv Sci (Weinh). 2024 Aug;11(31):e2402338. doi: 10.1002/advs.202402338. Epub 2024 Jun 14. Adv Sci (Weinh). 2024. PMID: 38874205 Free PMC article. Review.
References
-
- Bertalan G, Becker J, Tzschätzsch H, Morr A, Herthum H, Shahryari M, Greenhalgh RD, Guo J, Schröder L, Alzheimer C, et al. Mechanical behavior of the hippocampus and corpus callosum: An attempt to reconcile ex vivo with in vivo and micro with macro properties. J Mech Behav Biomed Mater. 2023;138:105613. doi: 10.1016/j.jmbbm.2022.105613. - DOI - PubMed
-
- Bertalan G, Klein C, Schreyer S, Steiner B, Kreft B, Tzschätzsch H, de Schellenberger AA, Nieminen-Kelhä M, Braun J, Guo J, et al. Biomechanical properties of the hypoxic and dying brain quantified by magnetic resonance elastography. Acta Biomater. 2020;101:395–402. doi: 10.1016/j.actbio.2019.11.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

