Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients With Type 2 Diabetes
- PMID: 38175642
- PMCID: PMC10767614
- DOI: 10.1001/jamanetworkopen.2023.50408
Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients With Type 2 Diabetes
Abstract
Importance: Concerns have been raised that glucagon-like peptide-1 receptor agonists (GLP-1RA) may increase the risk of pancreatic cancer.
Objective: To investigate the association of GLP-1RA treatment with pancreatic cancer incidence over 9 years of follow-up.
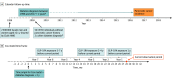
Design, setting, and participants: In this population-based historical cohort study, adult patients (aged 21 to 89 years) with type 2 diabetes insured by Clalit Healthcare Services, the largest state-mandated health organization in Israel, were followed up from 2009, when GLP-1RA became available in Israel, until pancreatic cancer diagnosis, death, reaching age 90 years, or end of follow-up (December 2017). Data were analyzed from June 2022 to November 2023.
Exposures: Treatment with GLP-1RA was compared with basal insulin.
Main outcome and measures: Pancreatic cancer incidence was compared according to weighted cumulative exposures to GLP-1RA and to basal insulin in a Cox model implemented in discrete time, with time origin at 2 years after diabetes diagnosis, adjusting for confounding. In sensitivity analyses, propensity score-matched pair new-user design and prevalent new-user design were used for the comparison. Because of risk for reverse-causation bias, results in the fifth to seventh year after medication were emphasized.
Results: During a cumulative follow-up of 3 290 439 person-years of 543 595 adults with a mean (SD) age of 59.9 (12.8) years (277 502 women [51%]) with incident diabetes, 1665 patients received pancreatic cancer diagnoses. In total, 33 377 patients (6.1%) used GLP-1RA and 106 849 (19.7%) used basal insulin. The estimated hazard ratio (HR) for pancreatic cancer associated with incremental use of 1 defined daily dose per day of GLP-1RA compared with basal insulin in the fifth to seventh year previously (all other characteristics, including age, sex, ethnic background, sociodemographic status, baseline body mass index, smoking history, history of pancreatitis, other glucose-lowering medications treatment history, and length of diabetes, being equal) was 0.50 (95% CI, 0.15-1.71). The new-user and prevalent new-user designs showed HRs from the fifth year onwards following initiation of GLP-1RA vs basal insulin of 0.52 (95 % CI, 0.19-1.41) and 0.75 (95 % CI, 0.37-1.53), respectively.
Conclusions and relevance: In this historical cohort study of adults with type 2 diabetes, no support for an increased pancreatic cancer incidence over 7 years following start of GLP-1RA treatment was found. However, monitoring for pancreatic cancer risk beyond 7 years following initiation of therapy is still required.
Trial registration: ClinicalTrials.gov Identifier: NCT02072902.
Conflict of interest statement
Figures
References
-
- Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173(7):534-539. doi:10.1001/jamainternmed.2013.2720 - DOI - PubMed
-
- US Food and Drug Administration . FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. 2016. Accessed November 29, 2023. https://www.fda.gov/Drugs/DrugSafety/ ucm343187.htm
-
- Vrang N, Jelsing J, Simonsen L, et al. . The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab. 2012;303(2):E253-E264. doi:10.1152/ajpendo.00182.2012 - DOI - PubMed

