CDK4/6-Inhibitors Versus Chemotherapy in Advanced HR+/HER2-Negative Breast Cancer: Results and Correlative Biomarker Analyses of the KENDO Randomized Phase II Trial
- PMID: 38175669
- PMCID: PMC11067809
- DOI: 10.1093/oncolo/oyad337
CDK4/6-Inhibitors Versus Chemotherapy in Advanced HR+/HER2-Negative Breast Cancer: Results and Correlative Biomarker Analyses of the KENDO Randomized Phase II Trial
Abstract
Background: The optimal treatment approach for hormone receptor-positive/HER2-negative metastatic breast cancer (HR+/HER2-negative MBC) with aggressive characteristics remains controversial, with lack of randomized trials comparing cyclin-dependent kinase (CDK)4/6-inhibitors (CDK4/6i) + endocrine therapy (ET) with chemotherapy + ET.
Materials and methods: We conducted an open-label randomized phase II trial (NCT03227328) to investigate whether chemotherapy + ET is superior to CDK4/6i + ET for HR+/HER2-negative MBC with aggressive features. PAM50 intrinsic subtypes (IS), immunological features, and gene expression were assessed on baseline samples.
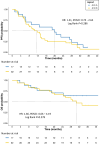
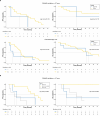
Results: Among 49 randomized patients (median follow-up: 35.2 months), median progression-free survival (mPFS) with chemotherapy + ET (11.2 months, 95% confidence interval [CI]: 7.7-15.4) was numerically shorter than mPFS (19.9 months, 95% CI: 9.0-30.6) with CDK4/6i + ET (hazard ratio: 1.41, 95% CI: 0.75-2.64). Basal-like tumors under CDK4/6i + ET exhibited worse PFS (mPFS: 11.4 months, 95% CI: 3.00-not reached [NR]) and overall survival (OS; mOS: 18.8 months, 95% CI: 18.8-NR) compared to other subtypes (mPFS: 20.7 months, 95% CI: 9.00-33.4; mOS: NR, 95% CI: 24.4-NR). In the chemotherapy arm, luminal A tumors showed poorer PFS (mPFS: 5.1 months, 95% CI: 2.7-NR) than other IS (mPFS: 13.2 months, 95% CI: 10.6-28.1). Genes/pathways involved in BC cell survival and proliferation were associated with worse outcomes, as opposite to most immune-related genes/signatures, especially in the CDK4/6i arm. CD24 was the only gene significantly associated with worse PFS in both arms. Tertiary lymphoid structures and higher tumor-infiltrating lymphocytes also showed favorable survival trends in the CDK4/6i arm.
Conclusions: The KENDO trial, although closed prematurely, adds further evidence supporting CDK4/6i + ET use in aggressive HR+/HER2-negative MBC instead of chemotherapy. PAM50 IS, genomic, and immunological features are promising biomarkers to personalize therapeutic choices.
Keywords: CDK4/6-inhibitors; breast cancer; chemotherapy; hormone receptors; intrinsic subtypes; tertiary lymphoid structures.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Francesco Schettini reports honoraria from Novartis, Gilead and Daiichy-Sankyo for educational events/materials and travel expenses from Novartis, Gilead and Daiichy-Sankyo. Michela Palleschi reports honoraria for educational events/materials from Novartis, Daiichy-Sankyo, Gilead and travel, accommodations, and/or expenses from Grant from Novartis. Ugo De Giorgi reports a consulting or advisory role for Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Dompé Farmaceutici, Eisai, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer, and PharmaMar; research funding (institution) from AstraZeneca, Roche, and Sanofi; and travel, accommodations, and/or expenses from Ipsen and Pfizer. Aleix Prat has received grants or contracts from Boehringer Ingelheim, Novartis, Roche, NanoString Technologies, Medica Scientia Innovation Research, Celgene, Astellas, and Pfizer; has received grants or contracts paid to his institution from Boehringer Ingelheim, Lilly, Roche, Novartis, Amgen, Daiichi Sankyo, and AstraZeneca; has received consulting fees from Roche, Pfizer, Novartis, Amgen, Bristol Myers Squibb, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc, Lilly, Daiichi Sankyo, and AstraZeneca; has received payment or honoraria from Roche, Pfizer, Novartis, Amgen, Bristol Myers Squibb, NanoString Technologies, and Daiichi Sankyo; has received payment or honoraria paid to his institution from NanoString Technologies; has participated on a data safety monitoring board or advisory board for Oncolytics Biotech, Lilly, AstraZeneca, Novartis, Peptomyc, and Roche; has served in a leadership or fiduciary role for Reveal Genomics and IOB Quironsalud; has stock ownership in Reveal Genomics, IOB Quironsalud, and Oncolytics Biotech; has served on the executive board for Reveal Genomics and SOLTI Cooperative Group; has served on the patronage committee for the SOLTI Foundation and Actitud Frente al Cáncer Foundation; and their institution has patents for HER2DX (pending), ERBB2 (issued), WO2018/103834A1, and CES WO/2018/096191. The other authors have nothing to declare.
Figures


Similar articles
-
Efficacy outcomes of CDK4/6 inhibitors in combination with endocrine therapy treatment in hormone receptor-positive/HER2-negative advanced breast cancer according to PAM50 intrinsic subtype: Primary results of SOLTI-1801 CDK-PREDICT study.Eur J Cancer. 2025 Feb 25;217:115219. doi: 10.1016/j.ejca.2024.115219. Epub 2025 Jan 3. Eur J Cancer. 2025. PMID: 39779447
-
Disparities in receipt of 1-st line CDK4/6 inhibitors with endocrine therapy for treatment of hormone receptor positive, HER2 negative metastatic breast cancer in the real-world setting.Breast Cancer Res. 2024 Oct 18;26(1):144. doi: 10.1186/s13058-024-01902-w. Breast Cancer Res. 2024. PMID: 39425174 Free PMC article.
-
Survival Following CDK4/6 Inhibitor Therapy for Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer.JAMA Netw Open. 2025 Feb 3;8(2):e2461067. doi: 10.1001/jamanetworkopen.2024.61067. JAMA Netw Open. 2025. PMID: 39982725 Free PMC article.
-
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i.BMC Cancer. 2024 May 23;24(1):631. doi: 10.1186/s12885-024-12269-8. BMC Cancer. 2024. PMID: 38783218 Free PMC article.
-
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
Cited by
-
Tertiary lymphoid structures in cancer: immune mechanisms and clinical implications.MedComm (2020). 2024 Mar 11;5(3):e489. doi: 10.1002/mco2.489. eCollection 2024 Mar. MedComm (2020). 2024. PMID: 38469550 Free PMC article. Review.
-
Activity of CDK4/6 inhibitors and parameters affecting survival in elderly patients in age-subgroups: Turkish Oncology Group (TOG) retrospective study.BMC Cancer. 2024 Dec 30;24(1):1592. doi: 10.1186/s12885-024-13357-5. BMC Cancer. 2024. PMID: 39736618 Free PMC article.
-
A translational journey into resistance: decoding the role of NF1 in CDK4/6-inhibitor failure.EBioMedicine. 2025 Aug;118:105860. doi: 10.1016/j.ebiom.2025.105860. Epub 2025 Jul 17. EBioMedicine. 2025. PMID: 40680385 Free PMC article. No abstract available.
-
A new insight into the impact of copy number variations on cell cycle deregulation of luminal-type breast cancer.Oncol Rev. 2025 Feb 12;19:1516409. doi: 10.3389/or.2025.1516409. eCollection 2025. Oncol Rev. 2025. PMID: 40017494 Free PMC article. Review.
-
Identifying predictors of treatment response and molecular changes induced by neoadjuvant chemotherapy and endocrine therapy in hormone receptor-positive/HER2-negative breast cancer: the NEOENDO translational study.ESMO Open. 2024 Dec;9(12):103989. doi: 10.1016/j.esmoop.2024.103989. Epub 2024 Nov 27. ESMO Open. 2024. PMID: 39608304 Free PMC article.
References
-
- Giuliano M, Schettini F, Rognoni C, et al.. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360-1369. 10.1016/S1470-2045(19)30420-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

