68Ga-FAPI PET imaging monitors response to combined TGF-βR inhibition and immunotherapy in metastatic colorectal cancer
- PMID: 38175716
- PMCID: PMC10866654
- DOI: 10.1172/JCI170490
68Ga-FAPI PET imaging monitors response to combined TGF-βR inhibition and immunotherapy in metastatic colorectal cancer
Abstract
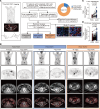
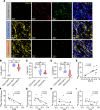
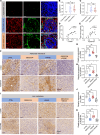
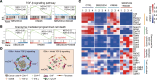
BACKGROUNDImproving and predicting tumor response to immunotherapy remains challenging. Combination therapy with a transforming growth factor-β receptor (TGF-βR) inhibitor that targets cancer-associated fibroblasts (CAFs) is promising for the enhancement of efficacy of immunotherapies. However, the effect of this approach in clinical trials is limited, requiring in vivo methods to better assess tumor responses to combination therapy.METHODSWe measured CAFs in vivo using the 68Ga-labeled fibroblast activation protein inhibitor-04 (68Ga-FAPI-04) for PET/CT imaging to guide the combination of TGF-β inhibition and immunotherapy. One hundred thirty-one patients with metastatic colorectal cancer (CRC) underwent 68Ga-FAPI and 18F-fluorodeoxyglucose (18F-FDG) PET/CT imaging. The relationship between uptake of 68Ga-FAPI and tumor immunity was analyzed in patients. Mouse cohorts of metastatic CRC were treated with the TGF-βR inhibitor combined with KN046, which blocks programmed death ligand 1 (PD-L1) and CTLA-4, followed by 68Ga-FAPI and 18F-FDG micro-PET/CT imaging to assess tumor responses.RESULTSPatients with metastatic CRC demonstrated high uptake rates of 68Ga-FAPI, along with suppressive tumor immunity and poor prognosis. The TGF-βR inhibitor enhanced tumor-infiltrating T cells and significantly sensitized metastatic CRC to KN046. 68Ga-FAPI PET/CT imaging accurately monitored the dynamic changes of CAFs and tumor response to combined the TGF-βR inhibitor with immunotherapy.CONCLUSION68Ga-FAPI PET/CT imaging is powerful in assessing tumor immunity and the response to immunotherapy in metastatic CRC. This study supports future clinical application of 68Ga-FAPI PET/CT to guide precise TGF-β inhibition plus immunotherapy in CRC patients, recommending 68Ga-FAPI and 18F-FDG dual PET/CT for CRC management.TRIAL REGISTRATIONCFFSTS Trial, ChiCTR2100053984, Chinese Clinical Trial Registry.FUNDINGNational Natural Science Foundation of China (82072695, 32270767, 82272035, 81972260).
Keywords: Cancer immunotherapy; Colorectal cancer; Diagnostic imaging; Gastroenterology; Oncology.
Figures




References
-
- Franko J, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. doi: 10.1016/S1470-2045(16)30500-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

