Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors
- PMID: 38175892
- PMCID: PMC12091338
- DOI: 10.1126/science.adh8342
Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors
Abstract
Immune checkpoint inhibitors can stimulate antitumor immunity but can also induce toxicities termed immune-related adverse events (irAEs). Colitis is a common and severe irAE that can lead to treatment discontinuation. Mechanistic understanding of gut irAEs has been hampered because robust colitis is not observed in laboratory mice treated with checkpoint inhibitors. We report here that this limitation can be overcome by using mice harboring the microbiota of wild-caught mice, which develop overt colitis following treatment with anti-CTLA-4 antibodies. Intestinal inflammation is driven by unrestrained activation of IFNγ-producing CD4+ T cells and depletion of peripherally induced regulatory T cells through Fcγ receptor signaling. Accordingly, anti-CTLA-4 nanobodies that lack an Fc domain can promote antitumor responses without triggering colitis. This work suggests a strategy for mitigating gut irAEs while preserving antitumor stimulating effects of CTLA-4 blockade.
Conflict of interest statement
Figures

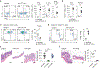


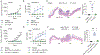
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

