Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid
- PMID: 38176203
- PMCID: PMC10801313
- DOI: 10.1016/j.ebiom.2023.104952
Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid
Abstract
Background: Gut probiotic depletion is associated with non-alcoholic fatty liver disease-associated hepatocellular carcinoma (NAFLD-HCC). Here, we investigated the prophylactic potential of Lactobacillus acidophilus against NAFLD-HCC.
Methods: NAFLD-HCC conventional and germ-free mice were established by diethylnitrosamine (DEN) injection with feeding of high-fat high-cholesterol (HFHC) or choline-deficient high-fat (CDHF) diet. Orthotopic NAFLD-HCC allografts were established by intrahepatic injection of murine HCC cells with HFHC feeding. Metabolomic profiling was performed using liquid chromatography-mass spectrometry. Biological functions of L. acidophilus conditional medium (L.a CM) and metabolites were determined in NAFLD-HCC human cells and mouse organoids.
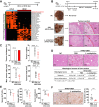
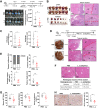
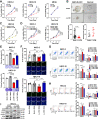
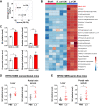
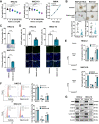
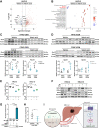
Findings: L. acidophilus supplementation suppressed NAFLD-HCC formation in HFHC-fed DEN-treated mice. This was confirmed in orthotopic allografts and germ-free tumourigenesis mice. L.a CM inhibited the growth of NAFLD-HCC human cells and mouse organoids. The protective function of L. acidophilus was attributed to its non-protein small molecules. By metabolomic profiling, valeric acid was the top enriched metabolite in L.a CM and its upregulation was verified in liver and portal vein of L. acidophilus-treated mice. The protective function of valeric acid was demonstrated in NAFLD-HCC human cells and mouse organoids. Valeric acid significantly suppressed NAFLD-HCC formation in HFHC-fed DEN-treated mice, accompanied by improved intestinal barrier integrity. This was confirmed in another NAFLD-HCC mouse model induced by CDHF diet and DEN. Mechanistically, valeric acid bound to hepatocytic surface receptor GPR41/43 to inhibit Rho-GTPase pathway, thereby ablating NAFLD-HCC.
Interpretation: L. acidophilus exhibits anti-tumourigenic effect in mice by secreting valeric acid. Probiotic supplementation is a potential prophylactic of NAFLD-HCC.
Funding: Shown in Acknowledgments.
Keywords: Cancer prevention; Lactobacillus acidophilus; Non-alcoholic fatty liver disease-associated hepatocellular carcinoma; Rho-GTPase pathway; Valeric acid.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors disclose no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

