Structural and functional basis of VLDLR usage by Eastern equine encephalitis virus
- PMID: 38176410
- PMCID: PMC10843625
- DOI: 10.1016/j.cell.2023.11.031
Structural and functional basis of VLDLR usage by Eastern equine encephalitis virus
Abstract
The very-low-density lipoprotein receptor (VLDLR) comprises eight LDLR type A (LA) domains and supports entry of distantly related alphaviruses, including Eastern equine encephalitis virus (EEEV) and Semliki Forest virus (SFV). Here, by resolving multiple cryo-electron microscopy structures of EEEV-VLDLR complexes and performing mutagenesis and functional studies, we show that EEEV uses multiple sites (E1/E2 cleft and E2 A domain) to engage more than one LA domain simultaneously. However, no single LA domain is necessary or sufficient to support efficient EEEV infection. Whereas all EEEV strains show conservation of two VLDLR-binding sites, the EEEV PE-6 strain and a few other EEE complex members feature a single amino acid substitution that enables binding of LA domains to an additional site on the E2 B domain. These structural and functional analyses informed the design of a minimal VLDLR decoy receptor that neutralizes EEEV infection and protects mice from lethal challenge.
Keywords: alphavirus; cryo-electron microscopy; encephalitis; mice; pathogenesis; receptor; therapeutic.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant or advisor for Inbios, Ocugen, Vir Biotechnology, Topspin Therapeutics, Moderna, Merck, and Immunome. The Diamond laboratory has received funding support from Emergent BioSolutions, Moderna, and Vir Biotechnology. D.H.F. is a founder of Courier Therapeutics and has received funding support from Emergent BioSolutions and Mallinckrodt Pharmaceuticals.
Figures
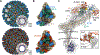





Update of
-
Structural and functional basis of VLDLR receptor usage by Eastern equine encephalitis virus.bioRxiv [Preprint]. 2023 Nov 15:2023.11.15.567188. doi: 10.1101/2023.11.15.567188. bioRxiv. 2023. Update in: Cell. 2024 Jan 18;187(2):360-374.e19. doi: 10.1016/j.cell.2023.11.031. PMID: 38014196 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

