Risk assessment and real-world outcomes in chronic thromboembolic pulmonary hypertension: insights from a UK pulmonary hypertension referral service
- PMID: 38176861
- PMCID: PMC10773408
- DOI: 10.1136/bmjopen-2023-080068
Risk assessment and real-world outcomes in chronic thromboembolic pulmonary hypertension: insights from a UK pulmonary hypertension referral service
Abstract
Objectives: This study was conducted to evaluate the ability of risk assessment to predict healthcare resource utilisation (HCRU), costs, treatments, health-related quality of life (HRQoL) and survival in patients diagnosed with chronic thromboembolic pulmonary hypertension (CTEPH).
Design: Retrospective observational study.
Setting: Pulmonary hypertension referral centre in the UK.
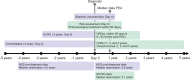
Participants: Adults diagnosed with CTEPH between 1 January 2012 and 30 June 2019 were included. Cohorts were retrospectively defined for operated patients (received pulmonary endarterectomy (PEA)) and not operated; further subgroups were defined based on risk score (low, intermediate or high risk for 1-year mortality) at diagnosis.
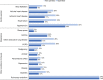
Primary and secondary outcome measures: Demographics, clinical characteristics, comorbidities, treatment patterns, HRQoL, HCRU, costs and survival outcomes were analysed.
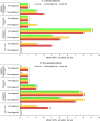
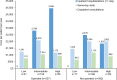
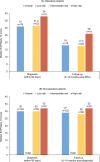
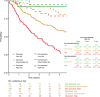
Results: Overall, 683 patients were analysed (268 (39%) operated; 415 (61%) not operated). Most patients in the operated and not-operated cohorts were intermediate risk (63%; 53%) or high risk (23%; 31%) at diagnosis. Intermediate-risk and high-risk patients had higher HCRU and costs than low-risk patients. Outpatient and accident and emergency visits were lower postdiagnosis for both cohorts and all risk groups versus prediagnosis. HRQoL scores noticeably improved in the operated cohort post-PEA, and less so in the not-operated cohort at 6-18 months postdiagnosis. Survival at 5 years was 83% (operated) and 49% (not operated) and was lower for intermediate-risk and high-risk patients compared with low-risk patients.
Conclusions: Findings from this study support that risk assessment at diagnosis is prognostic for mortality in patients with CTEPH. Low-risk patients have better survival and HRQoL and lower HCRU and costs compared with intermediate-risk and high-risk patients.
Keywords: Chronic airways disease; Mortality; Quality of Life.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DGK has received grants from Janssen Pharmaceuticals and Ferrer; consulting fees from Janssen Pharmaceuticals, MSD, Ferrer, Altavant and United Therapeutics; honoraria from Janssen Pharmaceuticals, MSD, Ferrer and United Therapeutics; funding from Janssen Pharmaceuticals, MSD and Ferrer to attend scientific meetings; has participated in a data safety monitoring board or Advisory Board for Janssen Pharmaceuticals and MSD; serves on the Specialist Respiratory Clinical Reference Group (unpaid) and as the UK National Audit Chair. NH has received honoraria payments from Janssen Pharmaceuticals, Vifor Pharmaceuticals and MSD. SW has received grants from Janssen Pharmaceuticals in support of the current study. CD has received a grant from Janssen, UK for investigator-led research unrelated to the present research, and a speaker’s honorarium from Janssen for an educational lecture. FE, LR and RM have no conflicts to disclose. AB, AM, RS and NP are employees of Actelion Pharmaceuticals. AB, AM and NP own stock in Johnson & Johnson. AL is supported by a British Heart Foundation Senior Basic Science Research Fellowship (FS/18/52/33808).
Figures

References
-
- Galiè N, Humbert M, Vachiery J-L, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
