Mild Deficits in Fear Learning: Evidence from Humans and Mice with Cerebellar Cortical Degeneration
- PMID: 38176906
- PMCID: PMC10897646
- DOI: 10.1523/ENEURO.0365-23.2023
Mild Deficits in Fear Learning: Evidence from Humans and Mice with Cerebellar Cortical Degeneration
Abstract
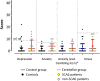
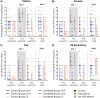
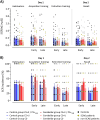
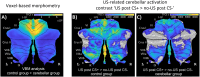
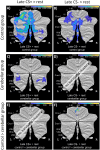
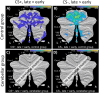
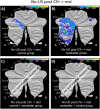
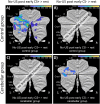
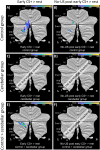
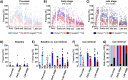
Functional brain imaging studies in humans suggest involvement of the cerebellum in fear conditioning but do not allow conclusions about the functional significance. The main aim of the present study was to examine whether patients with cerebellar degeneration show impaired fear conditioning and whether this is accompanied by alterations in cerebellar cortical activations. To this end, a 2 d differential fear conditioning study was conducted in 20 cerebellar patients and 21 control subjects using a 7 tesla (7 T) MRI system. Fear acquisition and extinction training were performed on day 1, followed by recall on day 2. Cerebellar patients learned to differentiate between the CS+ and CS-. Acquisition and consolidation of learned fear, however, was slowed. Additionally, extinction learning appeared to be delayed. The fMRI signal was reduced in relation to the prediction of the aversive stimulus and altered in relation to its unexpected omission. Similarly, mice with cerebellar cortical degeneration (spinocerebellar ataxia type 6, SCA6) were able to learn the fear association, but retrieval of fear memory was reduced. In sum, cerebellar cortical degeneration led to mild abnormalities in the acquisition of learned fear responses in both humans and mice, particularly manifesting postacquisition training. Future research is warranted to investigate the basis of altered fMRI signals related to fear learning.
Keywords: associative learning; cerebellar atrophy; cerebellum; fear conditioning; human; mouse model.
Copyright © 2024 Batsikadze et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
The cerebellum contributes to context-effects during fear extinction learning: A 7T fMRI study.Neuroimage. 2022 Jun;253:119080. doi: 10.1016/j.neuroimage.2022.119080. Epub 2022 Mar 9. Neuroimage. 2022. PMID: 35276369
-
Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain.Neurobiol Learn Mem. 2014 Dec;116:36-45. doi: 10.1016/j.nlm.2014.08.003. Epub 2014 Aug 13. Neurobiol Learn Mem. 2014. PMID: 25128878
-
Temporal dynamics of fMRI signal changes during conditioned interoceptive pain-related fear and safety acquisition and extinction.Behav Brain Res. 2022 Jun 3;427:113868. doi: 10.1016/j.bbr.2022.113868. Epub 2022 Mar 29. Behav Brain Res. 2022. PMID: 35364111
-
The anatomy of fear learning in the cerebellum: A systematic meta-analysis.Neurosci Biobehav Rev. 2015 Dec;59:83-91. doi: 10.1016/j.neubiorev.2015.09.019. Epub 2015 Oct 9. Neurosci Biobehav Rev. 2015. PMID: 26441374 Review.
-
The role of the basolateral amygdala and infralimbic cortex in (re)learning extinction.Psychopharmacology (Berl). 2019 Jan;236(1):303-312. doi: 10.1007/s00213-018-4957-x. Epub 2018 Jun 30. Psychopharmacology (Berl). 2019. PMID: 29959461 Review.
Cited by
-
Evidence for lateralization of fear emotions in the cerebellum.J Neurol. 2025 May 30;272(6):435. doi: 10.1007/s00415-025-13183-0. J Neurol. 2025. PMID: 40447788 Free PMC article. No abstract available.
References
-
- Adamaszek M, Manto M, Schutter DJL (2022) The emotional cerebellum, Ed 1. Switzerland: Springer.
MeSH terms
LinkOut - more resources
Full Text Sources
