15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β
- PMID: 38177096
- PMCID: PMC10766617
- DOI: 10.1038/s41467-023-43554-y
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β
Abstract
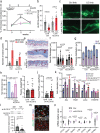
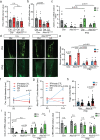
Lymphedema (LD) is characterized by the accumulation of interstitial fluid, lipids and inflammatory cell infiltrate in the limb. Here, we find that LD tissues from women who developed LD after breast cancer exhibit an inflamed gene expression profile. Lipidomic analysis reveals decrease in specialized pro-resolving mediators (SPM) generated by the 15-lipoxygenase (15-LO) in LD. In mice, the loss of SPM is associated with an increase in apoptotic regulatory T (Treg) cell number. In addition, the selective depletion of 15-LO in the lymphatic endothelium induces an aggravation of LD that can be rescued by Treg cell adoptive transfer or ALOX15-expressing lentivector injections. Mechanistically, exogenous injections of the pro-resolving cytokine IFN-β restores both 15-LO expression and Treg cell number in a mouse model of LD. These results provide evidence that lymphatic 15-LO may represent a therapeutic target for LD by serving as a mediator of Treg cell populations to resolve inflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors have submitted a patent application (application numbers EP22305165, EP22305165.7, inventor B. Garmy-Susini) based on the results reported in this study and the authors declare no other competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

