Mechanisms of immune checkpoint inhibitors: insights into the regulation of circular RNAS involved in cancer hallmarks
- PMID: 38177102
- PMCID: PMC10766988
- DOI: 10.1038/s41419-023-06389-5
Mechanisms of immune checkpoint inhibitors: insights into the regulation of circular RNAS involved in cancer hallmarks
Abstract
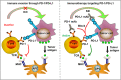
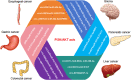
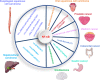
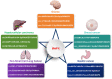
Current treatment strategies for cancer, especially advanced cancer, are limited and unsatisfactory. One of the most substantial advances in cancer therapy, in the last decades, was the discovery of a new layer of immunotherapy approach, immune checkpoint inhibitors (ICIs), which can specifically activate immune cells by targeting immune checkpoints. Immune checkpoints are a type of immunosuppressive molecules expressed on immune cells, which can regulate the degree of immune activation and avoid autoimmune responses. ICIs, such as anti-PD-1/PD-L1 drugs, has shown inspiring efficacy and broad applicability across various cancers. Unfortunately, not all cancer patients benefit remarkably from ICIs, and the overall response rates to ICIs remain relatively low for most cancer types. Moreover, the primary and acquired resistance to ICIs pose serious challenges to the clinical application of cancer immunotherapy. Thus, a deeper understanding of the molecular biological properties and regulatory mechanisms of immune checkpoints is urgently needed to improve clinical options for current therapies. Recently, circular RNAs (circRNAs) have attracted increasing attention, not only due to their involvement in various aspects of cancer hallmarks, but also for their impact on immune checkpoints in shaping the tumor immune microenvironment. In this review, we systematically summarize the current status of immune checkpoints in cancer and the existing regulatory roles of circRNAs on immune checkpoints. Meanwhile, we also aim to settle the issue in an evidence-oriented manner that circRNAs involved in cancer hallmarks regulate the effects and resistance of ICIs by targeting immune checkpoints.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

