Nuclear pore protein POM121 regulates subcellular localization and transcriptional activity of PPARγ
- PMID: 38177114
- PMCID: PMC10766976
- DOI: 10.1038/s41419-023-06371-1
Nuclear pore protein POM121 regulates subcellular localization and transcriptional activity of PPARγ
Abstract
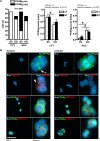
Manipulation of the subcellular localization of transcription factors by preventing their shuttling via the nuclear pore complex (NPC) emerges as a novel therapeutic strategy against cancer. One transmembrane component of the NPC is POM121, encoded by a tandem gene locus POM121A/C on chromosome 7. Overexpression of POM121 is associated with metabolic diseases (e.g., diabetes) and unfavorable clinical outcome in patients with colorectal cancer (CRC). Peroxisome proliferator-activated receptor-gamma (PPARγ) is a transcription factor with anti-diabetic and anti-tumoral efficacy. It is inhibited by export from the nucleus to the cytosol via the RAS-RAF-MEK1/2-ERK1/2 signaling pathway, a major oncogenic driver of CRC. We therefore hypothesized that POM121 participates in the transport of PPARγ across the NPC to regulate its transcriptional activity on genes involved in metabolic and tumor control. We found that POM121A/C mRNA was enriched and POM121 protein co-expressed with PPARγ in tissues from CRC patients conferring poor prognosis. Its interactome was predicted to include proteins responsible for tumor metabolism and immunity, and in-silico modeling provided insights into potential 3D structures of POM121. A peptide region downstream of the nuclear localization sequence (NLS) of POM121 was identified as a cytoplasmic interactor of PPARγ. POM121 positivity correlated with the cytoplasmic localization of PPARγ in patients with KRAS mutant CRC. In contrast, POM121A/C silencing by CRISPR/Cas9 sgRNA or siRNA enforced nuclear accumulation of PPARγ and activated PPARγ target genes promoting lipid metabolism and cell cycle arrest resulting in reduced proliferation of human CRC cells. Our data suggest the POM121-PPARγ axis as a potential drugable target in CRC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
NLS-mediated NPC functions of the nucleoporin Pom121.FEBS Lett. 2010 Aug 4;584(15):3292-8. doi: 10.1016/j.febslet.2010.07.008. Epub 2010 Jul 14. FEBS Lett. 2010. PMID: 20624389
-
Localization of Pom121 to the inner nuclear membrane is required for an early step of interphase nuclear pore complex assembly.Mol Biol Cell. 2011 Apr;22(7):1058-69. doi: 10.1091/mbc.E10-07-0641. Epub 2011 Feb 2. Mol Biol Cell. 2011. PMID: 21289085 Free PMC article.
-
Nuclear Pores Promote Lethal Prostate Cancer by Increasing POM121-Driven E2F1, MYC, and AR Nuclear Import.Cell. 2018 Aug 23;174(5):1200-1215.e20. doi: 10.1016/j.cell.2018.07.015. Epub 2018 Aug 9. Cell. 2018. PMID: 30100187 Free PMC article.
-
MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma.Cell Cycle. 2007 Jul 1;6(13):1539-48. doi: 10.4161/cc.6.13.4453. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17611413 Review.
-
Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) as a Target for Concurrent Management of Diabetes and Obesity-Related Cancer.Curr Pharm Des. 2017;23(25):3677-3688. doi: 10.2174/1381612823666170704125104. Curr Pharm Des. 2017. PMID: 28677503 Review.
Cited by
-
Advances in the understanding of nuclear pore complexes in human diseases.J Cancer Res Clin Oncol. 2024 Jul 30;150(7):374. doi: 10.1007/s00432-024-05881-5. J Cancer Res Clin Oncol. 2024. PMID: 39080077 Free PMC article. Review.
-
The Potential of Nuclear Pore Complexes in Cancer Therapy.Molecules. 2024 Oct 12;29(20):4832. doi: 10.3390/molecules29204832. Molecules. 2024. PMID: 39459201 Free PMC article. Review.
-
Multi-omics analysis and experimental verification reveal testicular fatty acid metabolism disorder in non-obstructive azoospermia.Zool Res. 2025 Jan 18;46(1):177-192. doi: 10.24272/j.issn.2095-8137.2024.223. Zool Res. 2025. PMID: 39846195 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- Bu2285/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GRK2727/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GRK2727/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ICON/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GRK2727/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

