A pilot optical coherence tomography angiography classification of retinal neovascularization in retinopathy of prematurity
- PMID: 38177160
- PMCID: PMC10766630
- DOI: 10.1038/s41598-023-49964-8
A pilot optical coherence tomography angiography classification of retinal neovascularization in retinopathy of prematurity
Abstract
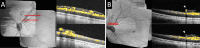
Extraretinal neovascularization is a hallmark of treatment-requiring retinopathy of prematurity (ROP). Optical coherence tomography angiography (OCTA) offers vascular flow and depth information not available from indirect ophthalmoscopy and structural OCT, but OCTA is only commercially available as a tabletop device. In this study, we used an investigational handheld OCTA device to study the vascular flow in and around retinal neovascularization in seven preterm infants with treatment-requiring ROP and contrasted them to images of vascular flow in six infants of similar age without neovascular ROP. We showed stages of retinal neovascularization visible in preterm infants from 32 to 47 weeks postmenstrual age: Intraretinal neovascularization did not break through the internal limiting membrane; Subclinical neovascular buds arose from retinal vasculature with active flow through the internal limiting membrane; Flat neovascularization in aggressive ROP assumed a low-lying configuration compared to elevated extraretinal neovascular plaques; Regressed neovascularization following treatment exhibited decreased vascular flow within the preretinal tissue, but flow persisted in segments of retinal vessels elevated from their original intraretinal location. These findings enable a pilot classification of retinal neovascularization in eyes with ROP using OCTA, and may be helpful in detailed monitoring of disease progression, treatment response and predicting reactivation.
© 2024. The Author(s).
Conflict of interest statement
Drs. Viehland, Izatt and Toth and Duke University have patent application pending related to the novel handheld probe used in the manuscript. Drs. Viehland and Toth have owner equity in Theia Imaging, LLC. No other authors have related conflict of interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

