Dietary- and host-derived metabolites are used by diverse gut bacteria for anaerobic respiration
- PMID: 38177297
- PMCID: PMC11055453
- DOI: 10.1038/s41564-023-01560-2
Dietary- and host-derived metabolites are used by diverse gut bacteria for anaerobic respiration
Erratum in
-
Author Correction: Dietary- and host-derived metabolites are used by diverse gut bacteria for anaerobic respiration.Nat Microbiol. 2025 Jun;10(6):1550. doi: 10.1038/s41564-025-02031-6. Nat Microbiol. 2025. PMID: 40379934 No abstract available.
Abstract
Respiratory reductases enable microorganisms to use molecules present in anaerobic ecosystems as energy-generating respiratory electron acceptors. Here we identify three taxonomically distinct families of human gut bacteria (Burkholderiaceae, Eggerthellaceae and Erysipelotrichaceae) that encode large arsenals of tens to hundreds of respiratory-like reductases per genome. Screening species from each family (Sutterella wadsworthensis, Eggerthella lenta and Holdemania filiformis), we discover 22 metabolites used as respiratory electron acceptors in a species-specific manner. Identified reactions transform multiple classes of dietary- and host-derived metabolites, including bioactive molecules resveratrol and itaconate. Products of identified respiratory metabolisms highlight poorly characterized compounds, such as the itaconate-derived 2-methylsuccinate. Reductase substrate profiling defines enzyme-substrate pairs and reveals a complex picture of reductase evolution, providing evidence that reductases with specificities for related cinnamate substrates independently emerged at least four times. These studies thus establish an exceptionally versatile form of anaerobic respiration that directly links microbial energy metabolism to the gut metabolome.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



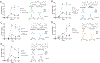


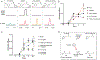







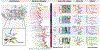
References
-
- Moodie AD & Ingledew WJ Microbial anaerobic respiration. Adv Microb Physiol 31, 225–69 (1990). - PubMed
-
- Gibson GR, Macfarlane GT & Cummings JH Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. Journal of Applied Bacteriology 65, 103–111 (1988). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

