Somatic mouse models of gastric cancer reveal genotype-specific features of metastatic disease
- PMID: 38177458
- PMCID: PMC10899107
- DOI: 10.1038/s43018-023-00686-w
Somatic mouse models of gastric cancer reveal genotype-specific features of metastatic disease
Abstract
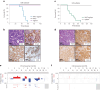
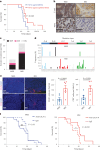
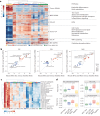
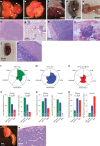
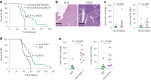
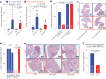
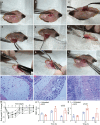
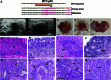
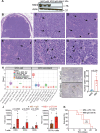
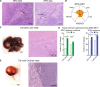
Metastatic gastric carcinoma is a highly lethal cancer that responds poorly to conventional and molecularly targeted therapies. Despite its clinical relevance, the mechanisms underlying the behavior and therapeutic response of this disease are poorly understood owing, in part, to a paucity of tractable models. Here we developed methods to somatically introduce different oncogenic lesions directly into the murine gastric epithelium. Genotypic configurations observed in patients produced metastatic gastric cancers that recapitulated the histological, molecular and clinical features of all nonviral molecular subtypes of the human disease. Applying this platform to both wild-type and immunodeficient mice revealed previously unappreciated links between the genotype, organotropism and immune surveillance of metastatic cells, which produced distinct patterns of metastasis that were mirrored in patients. Our results establish a highly portable platform for generating autochthonous cancer models with flexible genotypes and host backgrounds, which can unravel mechanisms of gastric tumorigenesis or test new therapeutic concepts.
© 2024. The Author(s).
Conflict of interest statement
While not directly related to this manuscript, S.W.L. declares outside consultancy and equity for Oric Pharmaceuticals, Blueprint Medicines, Mirimus, Senecea Therapeutics, Faeth Therapeutics and PMV Pharmaceuticals; and outside consultancy (no equity) for Fate Therapeutics. J.L. has received consulting fees from Mirimus. The other authors declare no competing interests. None of these affiliations represents a competing interest with respect to the design or execution of this study or interpretation of data presented in this report.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

