Identifying patterns in amyotrophic lateral sclerosis progression from sparse longitudinal data
- PMID: 38177466
- PMCID: PMC10766562
- DOI: 10.1038/s43588-022-00299-w
Identifying patterns in amyotrophic lateral sclerosis progression from sparse longitudinal data
Abstract
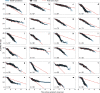
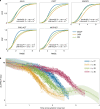
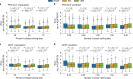
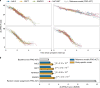
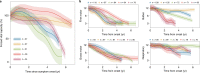
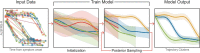
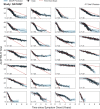
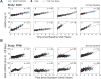
The clinical presentation of amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease, varies widely across patients, making it challenging to determine if potential therapeutics slow progression. We sought to determine whether there were common patterns of disease progression that could aid in the design and analysis of clinical trials. We developed an approach based on a mixture of Gaussian processes to identify clusters of patients sharing similar disease progression patterns, modeling their average trajectories and the variability in each cluster. We show that ALS progression is frequently nonlinear, with periods of stable disease preceded or followed by rapid decline. We also show that our approach can be extended to Alzheimer's and Parkinson's diseases. Our results advance the characterization of disease progression of ALS and provide a flexible modeling approach that can be applied to other progressive diseases.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
K.N., K. Severson and S.G. were employed by IBM Research during this project. K. Sachs consults for Modulo Bio Inc.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

