Versican accumulation drives Nos2 induction and aortic disease in Marfan syndrome via Akt activation
- PMID: 38177536
- PMCID: PMC10897446
- DOI: 10.1038/s44321-023-00009-7
Versican accumulation drives Nos2 induction and aortic disease in Marfan syndrome via Akt activation
Abstract
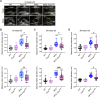
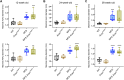
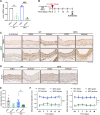
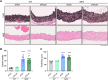
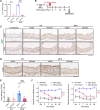
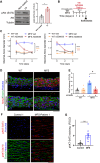
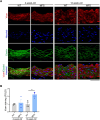
Thoracic aortic aneurysm and dissection (TAAD) is a life-threatening condition associated with Marfan syndrome (MFS), a disease caused by fibrillin-1 gene mutations. While various conditions causing TAAD exhibit aortic accumulation of the proteoglycans versican (Vcan) and aggrecan (Acan), it is unclear whether these ECM proteins are involved in aortic disease. Here, we find that Vcan, but not Acan, accumulated in Fbn1C1041G/+ aortas, a mouse model of MFS. Vcan haploinsufficiency protected MFS mice against aortic dilation, and its silencing reverted aortic disease by reducing Nos2 protein expression. Our results suggest that Acan is not an essential contributor to MFS aortopathy. We further demonstrate that Vcan triggers Akt activation and that pharmacological Akt pathway inhibition rapidly regresses aortic dilation and Nos2 expression in MFS mice. Analysis of aortic tissue from MFS human patients revealed accumulation of VCAN and elevated pAKT-S473 staining. Together, these findings reveal that Vcan plays a causative role in MFS aortic disease in vivo by inducing Nos2 via Akt activation and identify Akt signaling pathway components as candidate therapeutic targets.
Keywords: Akt; Aortic Aneurysm; Marfan Syndrome; Nos2; Versican.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome.Nat Med. 2017 Feb;23(2):200-212. doi: 10.1038/nm.4266. Epub 2017 Jan 9. Nat Med. 2017. PMID: 28067899
-
Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor β Signaling.J Am Heart Assoc. 2017 Jan 24;6(1):e004968. doi: 10.1161/JAHA.116.004968. J Am Heart Assoc. 2017. PMID: 28119285 Free PMC article.
-
Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection.JCI Insight. 2018 Mar 8;3(5):e97167. doi: 10.1172/jci.insight.97167. JCI Insight. 2018. PMID: 29515038 Free PMC article.
-
Pathophysiology and Therapeutics of Thoracic Aortic Aneurysm in Marfan Syndrome.Biomolecules. 2022 Jan 14;12(1):128. doi: 10.3390/biom12010128. Biomolecules. 2022. PMID: 35053276 Free PMC article. Review.
-
Nitric oxide in the Marfan vasculature: Friend or foe?Nitric Oxide. 2021 Nov 1;116:27-34. doi: 10.1016/j.niox.2021.08.006. Epub 2021 Aug 31. Nitric Oxide. 2021. PMID: 34478846 Review.
Cited by
-
Extracellular matrix in vascular homeostasis and disease.Nat Rev Cardiol. 2025 May;22(5):333-353. doi: 10.1038/s41569-024-01103-0. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743560 Review.
-
Genetic Manipulation of Caveolin-1 in a Transgenic Mouse Model of Aortic Root Aneurysm: Sex-Dependent Effects on Endothelial and Smooth Muscle Function.Int J Mol Sci. 2024 Nov 26;25(23):12702. doi: 10.3390/ijms252312702. Int J Mol Sci. 2024. PMID: 39684412 Free PMC article.
-
Identification of Pivotal ceRNA Networks Associated with Stanford-A Aortic Dissection via Integrated Bioinformatics Analysis.Int J Gen Med. 2025 Mar 17;18:1509-1527. doi: 10.2147/IJGM.S509177. eCollection 2025. Int J Gen Med. 2025. PMID: 40123810 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- PID2021-122388OB-100/Ministerio de Ciencia e Innovación (MCIN)
- PID2020-115217RB-100/Ministerio de Ciencia e Innovación (MCIN)
- RTI2018-099246-B-I00/Ministerio de Ciencia e Innovación (MCIN)
- CEX2021-001154-S/Ministerio de Ciencia e Innovación (MCIN)
- CEX2020-001041-S/Ministerio de Ciencia e Innovación (MCIN)
- BES-2016-077649/Ministerio de Ciencia e Innovación (MCIN)
- CD18/00028/Ministerio de Ciencia e Innovación (MCIN)
- IJC2020-044581-I/Ministerio de Ciencia e Innovación (MCIN)
- RYC2021-033343-I/Ministerio de Ciencia e Innovación (MCIN)
- HR18-00068/'la Caixa' Foundation ('la Caixa')
- CB16/11/00264/MEC | Instituto de Salud Carlos III (ISCIII)
- CB16/11/00479/MEC | Instituto de Salud Carlos III (ISCIII)
- PI17/00381/MEC | Instituto de Salud Carlos III (ISCIII)
- PI21/00084/MEC | Instituto de Salud Carlos III (ISCIII)
- 20151330/Fundació la Marató de TV3 (Fundació la Marató)
- INNVAL 21/24/Instituto de Investigación Marqués de Valdecilla (IDIVAL)
- MRF/1701/Marfan Foundation (The Marfan Foundation)
- Muévete por los que no pueden 2021/Fundacion MERCK-Fundacion Espanola de Enfermedades Raras
- FPU 20/04814/Ministerio de Universidades (MU)
LinkOut - more resources
Full Text Sources
Medical

