Resistance to BRAF inhibition explored through single circulating tumour cell molecular profiling in BRAF-mutant non-small-cell lung cancer
- PMID: 38177660
- PMCID: PMC10876548
- DOI: 10.1038/s41416-023-02535-0
Resistance to BRAF inhibition explored through single circulating tumour cell molecular profiling in BRAF-mutant non-small-cell lung cancer
Abstract
Background: Resistance mechanisms to combination therapy with dabrafenib plus trametinib remain poorly understood in patients with BRAFV600E-mutant advanced non-small-cell lung cancer (NSCLC). We examined resistance to BRAF inhibition by single CTC sequencing in BRAFV600E-mutant NSCLC.
Methods: CTCs and cfDNA were examined in seven BRAFV600E-mutant NSCLC patients at failure to treatment. Matched tumour tissue was available for four patients. Single CTCs were isolated by fluorescence-activated cell sorting following enrichment and immunofluorescence (Hoechst 33342/CD45/pan-cytokeratins) and sequenced for mutation and copy number-alteration (CNA) analyses.
Results: BRAFV600E was found in 4/4 tumour biopsies and 5/7 cfDNA samples. CTC mutations were mostly found in MAPK-independent pathways and only 1/26 CTCs were BRAFV600E mutated. CTC profiles encompassed the majority of matched tumour biopsy CNAs but 72.5% to 84.5% of CTC CNAs were exclusive to CTCs. Extensive diversity, involving MAPK, MAPK-related, cell cycle, DNA repair and immune response pathways, was observed in CTCs and missed by analyses on tumour biopsies and cfDNA. Driver alterations in clinically relevant genes were recurrent in CTCs.
Conclusions: Resistance was not driven by BRAFV600E-mutant CTCs. Extensive tumour genomic heterogeneity was found in CTCs compared to tumour biopsies and cfDNA at failure to BRAF inhibition, in BRAFV600E-mutant NSCLC, including relevant alterations that may represent potential treatment opportunities.
© 2024. The Author(s).
Conflict of interest statement
LM: Sponsored Research: Amgen, Bristol-Myers Squibb, Boehringer Ingelheim. Consulting, advisory role: Roche Diagnostics, Takeda, Roche. Lectures and educational activities: Bristol-Myers Squibb, Tecnofarma, Roche. Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche. Mentorship program with key opinion leaders: funded by AstraZeneca. BB: Sponsored Research at Gustave Roussy Cancer Center Abbvie, Amgen, AstraZeneca, Biogen, Blueprint Medicines, BMS, Celgene, Eli Lilly, GSK, Ignyta, IPSEN, Merck KGaA, MSD, Nektar, Onxeo, Pfizer, Pharma Mar, Sanofi, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma. DP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer. CME, Roche. Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Clinical trials research: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmune, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo. Travel, Accommodations, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer. The authors declare no competing interests.
Figures
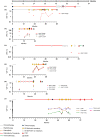




References
-
- Planchard D, Popat S, Kerr K, Novello S, Smit E, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annal Oncol. 2018;29: iv192–237. - PubMed
-
- Barlesi F, Mazières J, Merlio JP, Debieuvre D, Mosser J, Léna H, et al. Routine molecular profiling of cancer: results of a one-year nationwide program of the French Cooperative Thoracic Intergroup (IFCT) for advanced non-small cell lung cancer (NSCLC) patients. Lancet. 2016;287:1415–26. doi: 10.1016/S0140-6736(16)00004-0. - DOI - PubMed
-
- Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–93. doi: 10.1016/S1470-2045(16)30146-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

