Antimalarial artesunate-mefloquine versus praziquantel in African children with schistosomiasis: an open-label, randomized controlled trial
- PMID: 38177851
- PMCID: PMC10803269
- DOI: 10.1038/s41591-023-02719-4
Antimalarial artesunate-mefloquine versus praziquantel in African children with schistosomiasis: an open-label, randomized controlled trial
Abstract
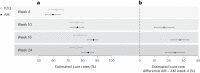
Schistosomiasis treatment entirely relies on a single drug, praziquantel, prompting research into alternative therapeutics. Here we evaluated the efficacy and safety of the antimalarial combination artesunate-mefloquine for the treatment of schistosomiasis in a proof-of-concept, pragmatic, open-label, randomized controlled trial in primary schools of six villages endemic for schistosomiasis in northern Senegal. Children (6-14 years) were eligible if Schistosoma eggs were detected by microscopy in urine and/or stool. In total, 726 children were randomized 1:1 to praziquantel (standard care: 40 mg kg-1 single dose; n = 364) or to artesunate-mefloquine (antimalarial dosage: artesunate 4 mg kg-1 and mefloquine 8 mg kg-1 daily for three consecutive days; n = 362). Eight children not meeting the inclusion criteria were excluded from efficacy analysis. Median age of the remaining 718 participants was 9 years; 399 (55.6%) were male, and 319 (44.4%) female; 99.3% were infected with Schistosoma haematobium and 15.2% with S. mansoni. Primary outcomes were cure rate, assessed by microscopy, and frequency of drug-related adverse effects of artesunate-mefloquine versus praziquantel at 4 weeks after treatment. Cure rate was 59.6% (208/349) in the artesunate-mefloquine arm versus 62.1% (211/340) in the praziquantel arm. The difference of -2.5% (95% confidence interval (CI) -9.8 to 4.8) met the predefined criteria of noninferiority (margin set at 10%). All drug-related adverse events were mild or moderate, and reported in 28/361 children receiving artesunate-mefloquine (7.8%; 95% CI 5.4 to 11.0) versus 8/363 (2.2%; 95% CI 1.1 to 4.3) receiving praziquantel (P < 0.001). Artesunate-mefloquine at antimalarial dosage was moderately safe and noninferior to standard-care praziquantel for the treatment of schistosomiasis, predominantly due to S. haematobium. Multicentric trials in different populations and epidemiological settings are needed to confirm these findings. ClinicalTrials.gov identifier: NCT03893097 .
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Evaluation of Artesunate-mefloquine as a Novel Alternative Treatment for Schistosomiasis in African Children (SchistoSAM): protocol of a proof-of-concept, open-label, two-arm, individually-randomised controlled trial.BMJ Open. 2021 Jun 24;11(6):e047147. doi: 10.1136/bmjopen-2020-047147. BMJ Open. 2021. PMID: 34168029 Free PMC article.
-
Praziquantel, mefloquine-praziquantel, and mefloquine-artesunate-praziquantel against Schistosoma haematobium: a randomized, exploratory, open-label trial.PLoS Negl Trop Dis. 2014 Jul 17;8(7):e2975. doi: 10.1371/journal.pntd.0002975. eCollection 2014 Jul. PLoS Negl Trop Dis. 2014. PMID: 25033291 Free PMC article. Clinical Trial.
-
Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial.Clin Infect Dis. 2010 May 1;50(9):1205-13. doi: 10.1086/651682. Clin Infect Dis. 2010. PMID: 20350194 Clinical Trial.
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD006404. doi: 10.1002/14651858.CD006404.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD006404. doi: 10.1002/14651858.CD006404.pub4. PMID: 30620055 Free PMC article. Updated.
-
Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD004529. doi: 10.1002/14651858.CD004529.pub3. Cochrane Database Syst Rev. 2021. PMID: 33459345 Free PMC article.
Cited by
-
Systems analysis unravels a common rural-urban gradient in immunological profile, function, and metabolic dependencies.Sci Adv. 2025 May 2;11(18):eadu0419. doi: 10.1126/sciadv.adu0419. Epub 2025 Apr 30. Sci Adv. 2025. PMID: 40305616 Free PMC article.
-
Efficacy and safety of praziquantel plus artemisinin-based combinations versus praziquantel in the treatment of Kenyan children with Schistosoma mansoni infection: open-label, randomized, head-to-head, non-inferiority trial.Antimicrob Agents Chemother. 2025 Feb 13;69(2):e0073924. doi: 10.1128/aac.00739-24. Epub 2024 Dec 19. Antimicrob Agents Chemother. 2025. PMID: 39699212 Free PMC article. Clinical Trial.
-
Schistosomiasis in the Military-A Narrative Review.Trop Med Infect Dis. 2024 Sep 19;9(9):221. doi: 10.3390/tropicalmed9090221. Trop Med Infect Dis. 2024. PMID: 39330910 Free PMC article. Review.
-
Artesunate: A Review of Its Potential Therapeutic Effects and Mechanisms in Digestive Diseases.Pharmaceutics. 2025 Feb 25;17(3):299. doi: 10.3390/pharmaceutics17030299. Pharmaceutics. 2025. PMID: 40142963 Free PMC article. Review.
-
Optimizing the therapeutic benefits of synriam combined with praziquantel in mice harbouring juvenile and mature Schistosoma mansoni.Sci Rep. 2025 Aug 7;15(1):28867. doi: 10.1038/s41598-025-14037-5. Sci Rep. 2025. PMID: 40775423 Free PMC article.
References
-
- Abajobir AA, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

