Belantamab mafodotin, pomalidomide and dexamethasone in refractory multiple myeloma: a phase 1/2 trial
- PMID: 38177852
- PMCID: PMC10878971
- DOI: 10.1038/s41591-023-02703-y
Belantamab mafodotin, pomalidomide and dexamethasone in refractory multiple myeloma: a phase 1/2 trial
Abstract
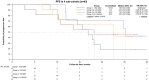
Due to evolving treatment standards for newly diagnosed multiple myeloma, many patients will be triple-class exposed after initial relapses and have poor survival. Novel therapies and combinations are therefore required to improve outcomes. B cell maturation antigen (BCMA)-targeted biologics have emerged as an important new area of therapeutics for relapsed multiple myeloma. The two-part ALGONQUIN trial evaluated various doses and schedules of the anti-BCMA antibody-drug conjugate belantamab mafodotin plus pomalidomide and dexamethasone for patients who are lenalidomide refractory and proteosome inhibitor exposed. The primary endpoints, including evaluating dose-limiting toxicities, establishing the recommended Part 2 dose (RP2D) and overall response rate for patients treated at the RP2D, were met. Secondary efficacy endpoints included progression-free survival and overall survival. Patients treated on study (N = 87) had a median of three previous regimens and 55.2% were triple-class refractory. At the RP2D the most common adverse events were decrease in best-corrected visual acuity (71.1%), keratopathy (65.8%), fatigue (57.9%), infection (47.4%; 7.9% grade ≥3), neutropenia (39.5%) and thrombocytopenia (39.5%). For RP2D patients (n = 38), the overall response rate was 85.3%, ≥very good partial response 75.7% and estimated two-year progression-free survival 52.8% (95% confidence interval, 33.9% to 82.4%), at a median follow-up of 13.9 months. The RP2D schedule was associated with manageable antibody-drug conjugate-associated corneal adverse events and improved tolerability without compromising efficacy. Belantamab mafodotin plus pomalidomide and dexamethasone induced durable responses with promising overall survival in relapsed multiple myeloma, the results of which are yet to be confirmed in the phase 3 DREAMM-8 study. ClinicalTrials.gov Identifier: NCT03715478 .
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: S.T. received grant support from GSK, Bristol Myers Squibb (BMS), Janssen, Pfizer, Amgen, Roche and Genentech; consulting fees from BMS, GSK and Roche; and honoraria from BMS, Janssen, Amgen, Sanofi and Pfizer. A.M. received honoraria from Celgene, Janssen, Amgen, Takeda, Sanofi and GSK. M.L.L. received research support from BMS/Celgene and Janssen; and honoraria from BMS/Celgene, Janssen, Gilead, AbbVie, AstraZeneca and Takeda. S.P. received consultancy honoraria from Janssen, BMS, FORUS and Apotex; study funding from GSK; and funding from BMS. D.W. received honoraria from Amgen, Antengene, BMS, FORUS Therapeutics, GSK, Janssen, Karyopharm, Pfizer, Sanofi and Takeda. M.P.C. received research support from BMS/Celgene and Janssen; and honoraria from BMS/Celgene, Janssen, Gilead, AbbVie, AstraZeneca and Takeda. R.K. received honoraria from Akcea Therapeutics, Amgen, BMS, Janssen, Merck, Sanofi, Celgene, Pfizer and Takeda; received research funding from Merck and Sanofi; and is a current equity holder in the private company Karyopharm. H.M. received advisory fees from Janssen, Takeda, Amgen, Pfizer, BMS, FORUS and Sanofi; received research funding from Janssen; and is supported by the early career award from Hamilton Health Sciences. I.O. participated in advisory boards for and received honoraria from Amgen, BMS, Celgene, FORUS Therapeutics, Janssen, Pfizer, Sanofi-Genzyme and Takeda; and received research funding from Janssen. J.S., A.K. and E.G. are employees of CMRG. D.R. received research funding and honoraria from, and is on the advisory board of, Janssen, BMS and Takeda; received research funding from Millennium Pharmaceuticals; and received honoraria from Amgen, Sanofi and GSK.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

