Sotorasib with panitumumab in chemotherapy-refractory KRASG12C-mutated colorectal cancer: a phase 1b trial
- PMID: 38177853
- PMCID: PMC11135132
- DOI: 10.1038/s41591-023-02717-6
Sotorasib with panitumumab in chemotherapy-refractory KRASG12C-mutated colorectal cancer: a phase 1b trial
Abstract
The current third-line (and beyond) treatment options for RAS-mutant metastatic colorectal cancer have yielded limited efficacy. At the time of study start, the combination of sotorasib, a KRAS (Kirsten rat sarcoma viral oncogene homolog)-G12C inhibitor, and panitumumab, an epidermal growth factor receptor (EGFR) inhibitor, was hypothesized to overcome treatment-induced resistance. This phase 1b substudy of the CodeBreaK 101 master protocol evaluated sotorasib plus panitumumab in patients with chemotherapy-refractory KRASG12C-mutated metastatic colorectal cancer. Here, we report the results in a dose-exploration cohort and a dose-expansion cohort. Patients received sotorasib (960 mg, once daily) plus panitumumab (6 mg kg-1, once every 2 weeks). The primary endpoints were safety and tolerability. Secondary endpoints included efficacy and pharmacokinetics. Exploratory biomarkers at baseline were assessed. Forty-eight patients (dose-exploration cohort, n = 8; dose-expansion cohort, n = 40) were treated. Treatment-related adverse events of any grade and grade ≥3 occurred in 45 (94%) and 13 (27%) patients, respectively. In the dose-expansion cohort, the confirmed objective response rate was 30.0% (95% confidence interval (CI) 16.6%, 46.5%). Median progression-free survival was 5.7 months (95% CI 4.2, 7.7 months). Median overall survival was 15.2 months (95% CI 12.5 months, not estimable). Prevalent genomic coalterations included APC (84%), TP53 (74%), SMAD4 (33%), PIK3CA (28%) and EGFR (26%). Sotorasib-panitumumab demonstrated acceptable safety with promising efficacy in chemotherapy-refractory KRASG12C-mutated metastatic colorectal cancer. ClinicalTrials.gov identifier: NCT04185883 .
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures

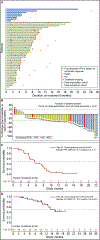
Comment in
-
KRAS and EGFR inhibitors: a new step in the management of colorectal cancer.Transl Gastroenterol Hepatol. 2024 Sep 4;9:56. doi: 10.21037/tgh-24-73. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39503022 Free PMC article. No abstract available.
-
KRAS G12C inhibitors in metastatic colorectal cancer: a tale of two targets.Transl Gastroenterol Hepatol. 2024 Aug 29;9:54. doi: 10.21037/tgh-24-48. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39503027 Free PMC article. No abstract available.
-
Precision medicine in metastatic colorectal cancer: targeting KRAS G12C mutation.Transl Gastroenterol Hepatol. 2024 Sep 12;9:53. doi: 10.21037/tgh-24-28. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39503041 Free PMC article. No abstract available.
References
-
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71, 209–249 (2021). - PubMed
-
- Siegel RL, Miller KD, Wagle NS & Jemal A Cancer statistics, 2023. 73, 17–48 (2023). - PubMed
-
- Benson AB, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 19, 329–359 (2021). - PubMed
-
- Grothey A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312 (2013). - PubMed
-
- Mayer RJ, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372, 1909–1919 (2015). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

