Gene therapy with bidridistrogene xeboparvovec for limb-girdle muscular dystrophy type 2E/R4: phase 1/2 trial results
- PMID: 38177855
- PMCID: PMC10803256
- DOI: 10.1038/s41591-023-02730-9
Gene therapy with bidridistrogene xeboparvovec for limb-girdle muscular dystrophy type 2E/R4: phase 1/2 trial results
Abstract
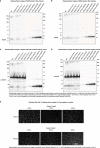
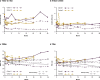
Limb-girdle muscular dystrophy 2E/R4 is caused by mutations in the β-sarcoglycan (SGCB) gene, leading to SGCB deficiency and consequent muscle loss. We developed a gene therapy approach based on functional replacement of the deficient SCB protein. Here we report interim results from a first-in-human, open-label, nonrandomized, phase 1/2 trial evaluating the safety and efficacy of bidridistrogene xeboparvovec, an adeno-associated virus-based gene therapy containing a codon-optimized, full-length human SGCB transgene. Patients aged 4-15 years with confirmed SGCB mutations at both alleles received one intravenous infusion of either 1.85 × 1013 vector genome copies kg-1 (Cohort 1, n = 3) or 7.41 × 1013 vector gene copies kg-1 (Cohort 2, n = 3). Primary endpoint was safety, and secondary endpoint was change in SGCB expression in skeletal muscle from baseline to Day 60. We report interim Year 2 results (trial ongoing). The most frequent treatment-related adverse events were vomiting (four of six patients) and gamma-glutamyl transferase increase (three of six patients). Serious adverse events resolved with standard therapies. Robust SGCB expression was observed: Day 60 mean (s.d.) percentage of normal expression 36.2% (2.7%) in Cohort 1 and 62.1% (8.7%) in Cohort 2. Post hoc exploratory analysis showed preliminary motor improvements using the North Star Assessment for Limb-girdle Type Muscular Dystrophies maintained through Year 2. The 2-year safety and efficacy of bidridistrogene xeboparvovec support clinical development advancement. Further studies are necessary to confirm the long-term safety and efficacy of this gene therapy. ClinicalTrials.gov registration: NCT03652259 .
© 2024. The Author(s).
Conflict of interest statement
J.R.M. received financial support from Sarepta Therapeutics, Inc. while at NCH at the time of the study and currently is an employee of Sarepta Therapeutics, Inc. E.R.P., S.L., D.A.G., S.N., R.P., X.L., H.S. and L.R.R.-K. are, or have been, employees of Sarepta Therapeutics, Inc. and may own stock/options in the company. L.P.L. and L.N.A. received fees from Sarepta Therapeutics, Inc. for licensure of the LGMD natural history dataset and served on the LGMD advisory board. L.P.L., L.N.A. and N.F.R. perform outcome measures training. B.S., K.J.L., K.C., N.F.R. and M.A.I. have no competing interests to declare and J.R.M with L.R.R.-K., are coinventors of bidridistrogene xeboparvovec.
Figures



References
-
- Wicklund MP. The limb-girdle muscular dystrophies. Continuum (Minneap. Minn.) 2019;25:1599–1618. - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

