A Comprehensive Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Drug Interactions of Nirmatrelvir/Ritonavir
- PMID: 38177893
- PMCID: PMC10786959
- DOI: 10.1007/s40262-023-01339-y
A Comprehensive Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Drug Interactions of Nirmatrelvir/Ritonavir
Abstract
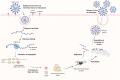
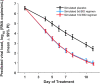
Nirmatrelvir is a potent and selective inhibitor of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease that is used as an oral antiviral coronavirus disease 2019 (COVID-19) treatment. To sustain unbound systemic trough concentrations above the antiviral in vitro 90% effective concentration value (EC90), nirmatrelvir is coadministered with 100 mg of ritonavir, a pharmacokinetic enhancer. Ritonavir inhibits nirmatrelvir's cytochrome P450 (CYP) 3A4-mediated metabolism which results in renal elimination becoming the primary route of nirmatrelvir elimination when dosed concomitantly. Nirmatrelvir exhibits absorption-limited nonlinear pharmacokinetics. When coadministered with ritonavir in patients with mild-to-moderate COVID-19, nirmatrelvir reaches a maximum concentration of 3.43 µg/mL (11.7× EC90) in approximately 3 h on day 5 of dosing, with a geometric mean day 5 trough concentration of 1.57 µg/mL (5.4× EC90). Drug interactions with nirmatrelvir/ritonavir (PAXLOVIDTM) are primarily attributed to ritonavir-mediated CYP3A4 inhibition, and to a lesser extent CYP2D6 and P-glycoprotein inhibition. Population pharmacokinetics and quantitative systems pharmacology modeling support twice daily dosing of 300 mg/100 mg nirmatrelvir/ritonavir for 5 days, with a reduced 150 mg/100 mg dose for patients with moderate renal impairment. Rapid clinical development of nirmatrelvir/ritonavir in response to the emerging COVID-19 pandemic was enabled by innovations in clinical pharmacology research, including an adaptive phase 1 trial design allowing direct to pivotal phase 3 development, fluorine nuclear magnetic resonance spectroscopy to delineate absorption, distribution, metabolism, and excretion profiles, and innovative applications of model-informed drug development to accelerate development.
© 2024. The Author(s).
Conflict of interest statement
All authors are employees of Pfizer Inc and may hold stock or stock options.
Figures

References
-
- Avezum Á, Oliveira GBF, Oliveira H, Lucchetta RC, Pereira VFA, Dabarian AL, et al. Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE - Coalition V): a double-blind, multicentre, randomised, controlled trial. Lancet Reg Health Am. 2022;11:100243. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

