Cannabidiol (Epidyolex®) for severe behavioral manifestations in patients with tuberous sclerosis complex, mucopolysaccharidosis type III and fragile X syndrome: protocol for a series of randomized, placebo-controlled N-of-1 trials
- PMID: 38177999
- PMCID: PMC10768432
- DOI: 10.1186/s12888-023-05422-3
Cannabidiol (Epidyolex®) for severe behavioral manifestations in patients with tuberous sclerosis complex, mucopolysaccharidosis type III and fragile X syndrome: protocol for a series of randomized, placebo-controlled N-of-1 trials
Abstract
Background: Many rare genetic neurodevelopmental disorders (RGNDs) are characterized by intellectual disability (ID), severe cognitive and behavioral impairments, potentially diagnosed as a comorbid autism spectrum disorder or attention-deficit hyperactivity disorder. Quality of life is often impaired due to irritability, aggression and self-injurious behavior, generally refractory to standard therapies. There are indications from previous (case) studies and patient reporting that cannabidiol (CBD) may be an effective treatment for severe behavioral manifestations in RGNDs. However, clear evidence is lacking and interventional research is challenging due to the rarity as well as the heterogeneity within and between disease groups and interindividual differences in treatment response. Our objective is to examine the effectiveness of CBD on severe behavioral manifestations in three RGNDs, including Tuberous Sclerosis Complex (TSC), mucopolysaccharidosis type III (MPS III), and Fragile X syndrome (FXS), using an innovative trial design.
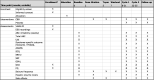
Methods: We aim to conduct placebo-controlled, double-blind, block-randomized, multiple crossover N-of-1 studies with oral CBD (twice daily) in 30 patients (aged ≥ 6 years) with confirmed TSC, MPS III or FXS and severe behavioral manifestations. The treatment is oral CBD up to a maximum of 25 mg/kg/day, twice daily. The primary outcome measure is the subscale irritability of the Aberrant Behavior Checklist. Secondary outcome measures include (personalized) patient-reported outcome measures with regard to behavioral and psychiatric outcomes, disease-specific outcome measures, parental stress, seizure frequency, and adverse effects of CBD. Questionnaires will be completed and study medication will be taken at the participants' natural setting. Individual treatment effects will be determined based on summary statistics. A mixed model analysis will be applied for analyzing the effectiveness of the intervention per disorder and across disorders combining data from the individual N-of-1 trials.
Discussion: These N-of-1 trials address an unmet medical need and will provide information on the effectiveness of CBD for severe behavioral manifestations in RGNDs, potentially generating generalizable knowledge at an individual-, disorder- and RGND population level.
Trial registration: EudraCT: 2021-003250-23, registered 25 August 2022, https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-003250-23/NL .
Keywords: Behavior; CBD; Cannabidiol; Fragile X syndrome; Intellectual disability; Mucopolysaccharidosis; N-of-1; Sanfilippo disease; Tuberous sclerosis complex.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Woittiez I, Putman L, Eggink E, Ras M, Sterkenburg PS. Zorg Beter Begrepen. Soc en Cult Planbureau. 2014; Available from: http://pdf.swphost.com/Sozio/e-nieuwsbrief/2014/Zorg_beter_begrepen_druk....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

