MBD3 promotes epithelial-mesenchymal transition in gastric cancer cells by upregulating ACTG1 via the PI3K/AKT pathway
- PMID: 38178023
- PMCID: PMC10768447
- DOI: 10.1186/s12575-023-00228-9
MBD3 promotes epithelial-mesenchymal transition in gastric cancer cells by upregulating ACTG1 via the PI3K/AKT pathway
Abstract
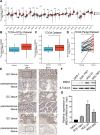
Background: Gastric cancer (GC) is a common malignancy and a leading cause of cancer-related death with high morbidity and mortality. Methyl-CpG binding domain protein 3 (MBD3), a key epigenetic regulator, is abnormally expressed in several cancers, participating in progression and metastasis. However, the role of MBD3 in GC remains unknown.
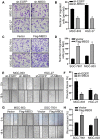
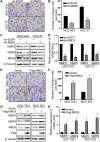
Methods: MBD3 expression was assessed via public databases and validated by western blotting and quantitative real-time polymerase chain reaction (qRT-PCR). The prognosis of MBD3 was analysed via bioinformatics based on the TCGA dataset. The migration, invasion and proliferation of GC cells were examined by transwell, wound healing, cell counting kit (CCK)-8, colony-formation and xenograft mouse models. Epithelial-mesenchymal transition (EMT) and phosphatidylinositide 3-kinases/ protein Kinase B (PI3K/AKT) pathway markers were evaluated by Western blotting. RNA sequencing was used to identify the target of MBD3.
Results: MBD3 expression was higher in GC tissues and cells than in normal tissues and cells. Additionally, high MBD3 levels were associated with poor prognosis in GC patients. Subsequently, we proved that MBD3 enhanced the migration, invasion and proliferation abilities of GC cells. Moreover, western blot results showed that MBD3 promoted EMT and activated the PI3K/AKT pathway. RNA sequencing analysis showed that MBD3 may increase actin γ1 (ACTG1) expression to promote migration and proliferation in GC cells.
Conclusion: MBD3 promoted migration, invasion, proliferation and EMT by upregulating ACTG1 via PI3K/AKT signaling activation in GC cells and may be a potential diagnostic and prognostic target.
Keywords: ACTG1; EMT; Gastric cancer; MBD3; PI3K/AKT.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
- KYCX22_3711/Postgraduate Research & Practice Innovation Program of Jiangsu Province
- 82072754/The National Natural Science Foundation of China
- M2020011/Research Project of Jiangsu Health and Health Commission
- BE2018689/Jiangsu Provincial Key Research and Development Program, China
- SH2018033/Zhenjiang Key Research and Development Program, China
LinkOut - more resources
Full Text Sources
Miscellaneous

