Doxycycline for transgene control disrupts gut microbiome diversity without compromising acute neuroinflammatory response
- PMID: 38178148
- PMCID: PMC10765643
- DOI: 10.1186/s12974-023-03004-4
Doxycycline for transgene control disrupts gut microbiome diversity without compromising acute neuroinflammatory response
Abstract
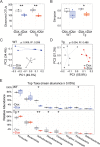
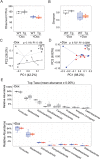
The tetracycline transactivator (tTA) system provides controllable transgene expression through oral administration of the broad-spectrum antibiotic doxycycline. Antibiotic treatment for transgene control in mouse models of disease might have undesirable systemic effects resulting from changes in the gut microbiome. Here we assessed the impact of doxycycline on gut microbiome diversity in a tTA-controlled model of Alzheimer's disease and then examined neuroimmune effects of these microbiome alterations following acute LPS challenge. We show that doxycycline decreased microbiome diversity in both transgenic and wild-type mice and that these changes persisted long after drug withdrawal. Despite the change in microbiome composition, doxycycline treatment had minimal effect on basal transcriptional signatures of inflammation the brain or on the neuroimmune response to LPS challenge. Our findings suggest that central neuroimmune responses may be less affected by doxycycline at doses needed for transgene control than by antibiotic cocktails at doses used for experimental microbiome disruption.
Keywords: APP transgenic mouse; Amyloid; Antibiotic; Doxycycline; Gut microbiome; LPS; Lipopolysaccharide; Microglia; Neuroinflammation; Tetracycline transactivator; Transcriptome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
The effect of doxycycline on alcohol consumption and sensitivity: consideration for inducible transgenic mouse models.Exp Biol Med (Maywood). 2012 Oct;237(10):1129-33. doi: 10.1258/ebm.2012.012029. Epub 2012 Sep 27. Exp Biol Med (Maywood). 2012. PMID: 23019604
-
Developing tTA transgenic rats for inducible and reversible gene expression.Int J Biol Sci. 2009;5(2):171-81. doi: 10.7150/ijbs.5.171. Epub 2009 Jan 29. Int J Biol Sci. 2009. PMID: 19214245 Free PMC article.
-
Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus.J Virol. 2002 Nov;76(22):11605-11. doi: 10.1128/jvi.76.22.11605-11611.2002. J Virol. 2002. PMID: 12388721 Free PMC article.
-
Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling.Semin Cell Dev Biol. 2002 Apr;13(2):121-8. doi: 10.1016/s1084-9521(02)00018-6. Semin Cell Dev Biol. 2002. PMID: 12127145 Review.
-
Challenges of repurposing tetracyclines for the treatment of Alzheimer's and Parkinson's disease.J Neural Transm (Vienna). 2022 Jun;129(5-6):773-804. doi: 10.1007/s00702-021-02457-2. Epub 2022 Jan 4. J Neural Transm (Vienna). 2022. PMID: 34982206 Review.
Cited by
-
Assessment of Antimicrobial Therapy in Eradicating Chlamydia muridarum in Research Mice: Immune Status and Its Impact on Outcomes.J Am Assoc Lab Anim Sci. 2025 Jan 1;64(1):76-88. doi: 10.30802/AALAS-JAALAS-24-069. J Am Assoc Lab Anim Sci. 2025. PMID: 40035285 Free PMC article.
-
Borreliosis and doxycycline treatment disrupt gut microbiota and immune responses in nonhuman primates.mBio. 2025 Aug 13;16(8):e0143725. doi: 10.1128/mbio.01437-25. Epub 2025 Jun 27. mBio. 2025. PMID: 40576342 Free PMC article.
-
Vasoactive Intestinal Peptide Receptor, CRTH2, Antagonist Treatment Improves Eosinophil and Mast Cell-Mediated Esophageal Remodeling and Motility Dysfunction in Eosinophilic Esophagitis.Cells. 2024 Feb 6;13(4):295. doi: 10.3390/cells13040295. Cells. 2024. PMID: 38391908 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

